Exporting Homogeneous Transition Metal Catalysts to Biological Habitats
- PMID: 36248016
- PMCID: PMC9542366
- DOI: 10.1002/ejoc.202200118
Exporting Homogeneous Transition Metal Catalysts to Biological Habitats
Abstract
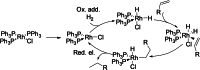
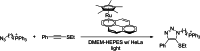
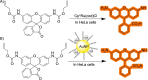
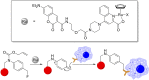
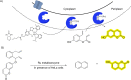
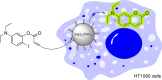
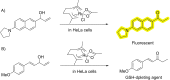
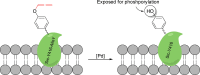
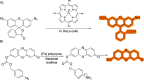
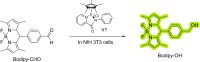
The possibility of performing designed transition-metal catalyzed reactions in biological and living contexts can open unprecedented opportunities to interrogate and interfere with biology. However, the task is far from obvious, in part because of the presumed incompatibly between organometallic chemistry and complex aqueous environments. Nonetheless, in the past decade there has been a steady progress in this research area, and several transition-metal (TM)-catalyzed bioorthogonal and biocompatible reactions have been developed. These reactions encompass a wide range of mechanistic profiles, which are very different from those used by natural metalloenzymes. Herein we present a summary of the latest progress in the field of TM-catalyzed bioorthogonal reactions, with a special focus on those triggered by activation of multiple carbon-carbon bonds.
Keywords: Biocatalysis; Bioorthogonal; Cell biology; Chemical biology; Organometallic catalysis.
© 2022 The Authors. European Journal of Organic Chemistry published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
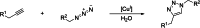
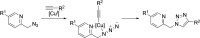

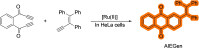
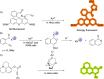
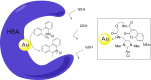
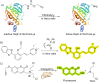



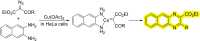
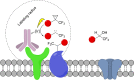
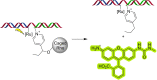
References
-
- D. L. Nelson, M. M. Cox in Lehninger Principles of Biochemistry, 7th ed., W. H. Freeman, New York, 2017.
-
- Sigel R. K. O., Pyle A. M., Chem. Rev. 2007, 107, 97–113. - PubMed
-
- None
-
- Rabinovich D. in Compr. Organomet. Chem. III (Eds. Mingos M. P., Crabtre R. H.). Elsevier, 2007, 1, 93–117;
-
- Grate J. W., Frye G. C. in Sensors Update, Vol. 2 (Eds.: Baltes H., Göpel W., Hesse J.), Wiley-VCH, Weinheim, 1996, pp. 10–20.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
