The promising role of hypoxia-resistant insulin-producing cells in ameliorating diabetes mellitus in vivo
- PMID: 36248064
- PMCID: PMC9540411
- DOI: 10.2144/fsoa-2022-0005
The promising role of hypoxia-resistant insulin-producing cells in ameliorating diabetes mellitus in vivo
Abstract
Aim: This study aimed to evaluate the efficacy of hypoxia-persistent insulin-producing cells (IPCs) against diabetes in vivo.
Materials & methods: Mesenchymal stem cells (MSCs) differentiation into IPCs in the presence of Se/Ti (III) or CeO2 nanomaterials. IPCs were subjected to hypoxia and hypoxia genes were analyzed. PKH-26-labeled IPCs were infused in diabetic rats to evaluate their anti-diabetic potential.
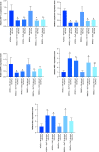
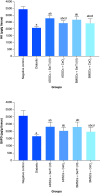
Results: MSCs were differentiated into functional IPCs. IPCs exhibited overexpression of anti-apoptotic genes and down-expression of hypoxia and apoptotic genes. IPCs implantation elicited glucose depletion and elevated insulin, HK and G6PD levels. They provoked VEGF and PDX-1 upregulation and HIF-1α and Caspase-3 down-regulation. IPCs transplantation ameliorated the destabilization of pancreatic tissue architecture.
Conclusion: The chosen nanomaterials were impressive in generating hypoxia-resistant IPCs that could be an inspirational strategy for curing diabetes.
Keywords: diabetes mellitus; hypoxia; insulin-producing cells; mesenchymal stem cells; nanomaterials.
© 2022 The Authors.
Figures





References
-
- Saeedi P, Petersohn I, Salpea P et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 157 (2019). . - PubMed
-
- Shelbaya S, Halawa M, Nasr M. The management of care of Egyptian patients with diabetes: a report from the International Diabetes Management Practices Study Wave 7. Med J Cairo Univ. 88(6), 1413–1421 (2020).
-
- Marcovecchio ML. Complications of acute and chronic hyperglycemia. US Endocrinol. 13(1), 17–21 (2017).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
