Protein Tyrosine Phosphatase Biochemical Inhibition Assays
- PMID: 36248604
- PMCID: PMC9516250
- DOI: 10.21769/BioProtoc.4510
Protein Tyrosine Phosphatase Biochemical Inhibition Assays
Abstract
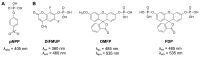
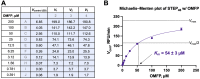
Disturbance of the dynamic balance between protein tyrosine phosphorylation and dephosphorylation, modulated by protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is known to be crucial for the development of many human diseases. The discovery of agents that restore this balance has been the subject of many drug research efforts, most of which have focused on tyrosine kinase inhibitors (TKIs), resulting in the development of more than 50 FDA-approved TKIs during the past two decades. More recently, accumulating evidence has suggested that members of the PTP superfamily are also promising drug targets, and efforts to discover tyrosine phosphatase inhibitors (TPIs) have increased dramatically. Here, we provide protocols for determining the potency of TPIs in vitro. We focus on the use of fluorescence-based substrates, which exhibit a dramatic increase in fluorescence emission when dephosphorylated by the PTP, and thus allow setting up highly sensitive and miniaturized phosphatase activity assays using 384-well or 1536-well microplates and a continuous (kinetic) assay format. The protocols cover PTP specific activity assays, Michaelis-Menten kinetics, dose-response inhibition assays, and dose-response data analysis for determining IC 50 values. Potential pitfalls are also discussed. While advanced instrumentation is utilized for compound spotting and liquid dispensing, all the assays can be adapted to existing equipment in most laboratories. Assays are described for selected PTP drug targets, including SHP2 ( PTPN11 ), PTP1B ( PTPN1 ), STEP ( PTPN5 ), and VHR ( DUSP3 ). However, all protocols are applicable to members of the PTP enzyme family in general. Graphical abstract.
Keywords: DUSP; Dose-response assay; IC 50; Inhibitor; Michaelis–Menten; PTP1B; Protein tyrosine phosphatase; SHP2; VHR.
Copyright © 2022 The Authors; exclusive licensee Bio-protocol LLC.
Conflict of interest statement
Competing interests The authors declare that they have no conflicts of interest with the contents of this article.
Figures









References
-
- Bessey O. A. , Lowry O. H. and Brock M. J. ( 1946 . ). A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum . J Biol Chem 164 : 321 - 329 . - PubMed
-
- Chen Y. N. , LaMarche M. J. , Chan H. M. , Fekkes P. , Garcia-Fortanet J. , Acker M. G. , Antonakos B. , Chen C. H. , Chen Z. , Cooke V. G. , et al. .( 2016 . ). Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases . Nature 535 ( 7610 ): 148 - 152 . - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

