Systems biology and artificial intelligence analysis highlights the pleiotropic effect of IVIg therapy in autoimmune diseases with a predominant role on B cells and complement system
- PMID: 36248801
- PMCID: PMC9563374
- DOI: 10.3389/fimmu.2022.901872
Systems biology and artificial intelligence analysis highlights the pleiotropic effect of IVIg therapy in autoimmune diseases with a predominant role on B cells and complement system
Abstract
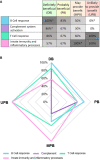
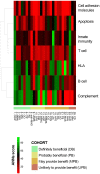
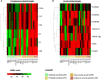
Intravenous immunoglobulin (IVIg) is used as treatment for several autoimmune and inflammatory conditions, but its specific mechanisms are not fully understood. Herein, we aimed to evaluate, using systems biology and artificial intelligence techniques, the differences in the pathophysiological pathways of autoimmune and inflammatory conditions that show diverse responses to IVIg treatment. We also intended to determine the targets of IVIg involved in the best treatment response of the evaluated diseases. Our selection and classification of diseases was based on a previously published systematic review, and we performed the disease characterization through manual curation of the literature. Furthermore, we undertook the mechanistic evaluation with artificial neural networks and pathway enrichment analyses. A set of 26 diseases was selected, classified, and compared. Our results indicated that diseases clearly benefiting from IVIg treatment were mainly characterized by deregulated processes in B cells and the complement system. Indeed, our results show that proteins related to B-cell and complement system pathways, which are targeted by IVIg, are involved in the clinical response. In addition, targets related to other immune processes may also play an important role in the IVIg response, supporting its wide range of actions through several mechanisms. Although B-cell responses and complement system have a key role in diseases benefiting from IVIg, protein targets involved in such processes are not necessarily the same in those diseases. Therefore, IVIg appeared to have a pleiotropic effect that may involve the collaborative participation of several proteins. This broad spectrum of targets and 'non-specificity' of IVIg could be key to its efficacy in very different diseases.
Keywords: B cells; IVIg Immunoglobulins; autoimmune diseases; complement system; immunomodulation; inflammatory diseases; intravenous immunoglobulin; mathematical models.
Copyright © 2022 Segú-Vergés, Caño, Calderón-Gómez, Bartra, Sardon, Kaveri and Terencio.
Conflict of interest statement
EC-G, SC, and JT are full time employees at Grifols. CS-V and HB are full time employees at Anaxomics Biotech. TS was a full time-employee at Anaxomics Biotech at the time of the study. SK has received fees for lectures and reviewing research proposals from CSL-Behring and Grifols. The authors declare that this study received funding from Grifols (including medical writing assistance, and the article processing fees). The funder participated in the original idea, writing of this article and decision to submit it for publication, but was not involved in the study design, collection, analysis and interpretation of data.
Figures

Similar articles
-
A machine learning analysis to predict the response to intravenous and subcutaneous immunoglobulin in inflammatory myopathies. A proposal for a future multi-omics approach in autoimmune diseases.Autoimmun Rev. 2022 Jun;21(6):103105. doi: 10.1016/j.autrev.2022.103105. Epub 2022 Apr 19. Autoimmun Rev. 2022. PMID: 35452850 Review.
-
Anti-inflammatory activity of intravenous immunoglobulin through scavenging of heme.Mol Immunol. 2019 Jul;111:205-208. doi: 10.1016/j.molimm.2019.04.020. Epub 2019 May 10. Mol Immunol. 2019. PMID: 31078967 Free PMC article.
-
Immunomodulation of autoimmune and inflammatory diseases with intravenous immunoglobulin.Clin Exp Med. 2005 Dec;5(4):135-40. doi: 10.1007/s10238-005-0079-y. Clin Exp Med. 2005. PMID: 16362793 Review.
-
Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases.Transfus Clin Biol. 2003 Jun;10(3):165-9. doi: 10.1016/s1246-7820(03)00035-1. Transfus Clin Biol. 2003. PMID: 12798851 Review.
-
[Mechanisms of action of intravenous immunoglobulins in the treatment of autoimmune diseases].Ann Med Interne (Paris). 1993;144(8):506-13. Ann Med Interne (Paris). 1993. PMID: 8179238 Review. French.
Cited by
-
MMPred: a tool to predict peptide mimicry events in MHC class II recognition.Front Genet. 2024 Dec 10;15:1500684. doi: 10.3389/fgene.2024.1500684. eCollection 2024. Front Genet. 2024. PMID: 39722794 Free PMC article.
-
Antibody diversity in IVIG: Therapeutic opportunities for novel immunotherapeutic drugs.Front Immunol. 2023 Mar 28;14:1166821. doi: 10.3389/fimmu.2023.1166821. eCollection 2023. Front Immunol. 2023. PMID: 37063852 Free PMC article. Review.
-
Restoring immune tolerance in pemphigus vulgaris.Proc Natl Acad Sci U S A. 2024 Jan 30;121(5):e2317762121. doi: 10.1073/pnas.2317762121. Epub 2024 Jan 23. Proc Natl Acad Sci U S A. 2024. PMID: 38261616 Free PMC article.
-
Study on the Treatment of ITP Mice with IVIG Sourced from Distinct Sex-Special Plasma (DSP-IVIG).Int J Mol Sci. 2023 Nov 6;24(21):15993. doi: 10.3390/ijms242115993. Int J Mol Sci. 2023. PMID: 37958975 Free PMC article.
-
Immunomodulatory and anti-inflammatory properties of immunoglobulin G antibodies.Immunol Rev. 2024 Nov;328(1):372-386. doi: 10.1111/imr.13404. Epub 2024 Sep 27. Immunol Rev. 2024. PMID: 39340138 Free PMC article. Review.
References
-
- European Medicines Agency . Guideline on core SmPC for human normal immunoglobulin for intravenous administration (IVIg) (2020). Available at: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guidel....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

