CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies
- PMID: 36248810
- PMCID: PMC9557333
- DOI: 10.3389/fimmu.2022.1019115
CAR-T cell therapy for hematological malignancies: Limitations and optimization strategies
Abstract
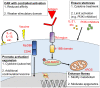
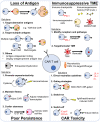
In the past decade, the emergence of chimeric antigen receptor (CAR) T-cell therapy has led to a cellular immunotherapy revolution against various cancers. Although CAR-T cell therapies have demonstrated remarkable efficacy for patients with certain B cell driven hematological malignancies, further studies are required to broaden the use of CAR-T cell therapy against other hematological malignancies. Moreover, treatment failure still occurs for a significant proportion of patients. CAR antigen loss on cancer cells is one of the most common reasons for cancer relapse. Additionally, immune evasion can arise due to the hostile immunosuppressive tumor microenvironment and the impaired CAR-T cells in vivo persistence. Other than direct antitumor activity, the adverse effects associated with CAR-T cell therapy are another major concern during treatment. As a newly emerged treatment approach, numerous novel preclinical studies have proposed different strategies to enhance the efficacy and attenuate CAR-T cell associated toxicity in recent years. The major obstacles that impede promising outcomes for patients with hematological malignancies during CAR-T cell therapy have been reviewed herein, along with recent advancements being made to surmount them.
Keywords: CAR-T cell therapy; hematological malignancies; immune evasion; toxicity; tumor microenvironment.
Copyright © 2022 Huang, Huang and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CAR-T Cell Therapy in Hematological Malignancies: Current Opportunities and Challenges.Front Immunol. 2022 Jun 10;13:927153. doi: 10.3389/fimmu.2022.927153. eCollection 2022. Front Immunol. 2022. PMID: 35757715 Free PMC article. Review.
-
CAR-T cell therapy in hematological malignancies: Where are we now and where are we heading for?Eur J Haematol. 2024 Jan;112(1):6-18. doi: 10.1111/ejh.14076. Epub 2023 Aug 7. Eur J Haematol. 2024. PMID: 37545253 Review.
-
Recent findings on chimeric antigen receptor (CAR)-engineered immune cell therapy in solid tumors and hematological malignancies.Stem Cell Res Ther. 2022 Sep 24;13(1):482. doi: 10.1186/s13287-022-03163-w. Stem Cell Res Ther. 2022. PMID: 36153626 Free PMC article. Review.
-
Strategies for Reducing Toxicity and Enhancing Efficacy of Chimeric Antigen Receptor T Cell Therapy in Hematological Malignancies.Int J Mol Sci. 2023 May 23;24(11):9115. doi: 10.3390/ijms24119115. Int J Mol Sci. 2023. PMID: 37298069 Free PMC article. Review.
-
Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies.Cancer Med. 2023 Apr;12(7):7844-7858. doi: 10.1002/cam4.5551. Epub 2022 Dec 30. Cancer Med. 2023. PMID: 36583504 Free PMC article. Review.
Cited by
-
From TCR fundamental research to innovative chimeric antigen receptor design.Nat Rev Immunol. 2025 Mar;25(3):212-224. doi: 10.1038/s41577-024-01093-7. Epub 2024 Oct 21. Nat Rev Immunol. 2025. PMID: 39433885 Review.
-
Advancements in the impact of human microbiota and probiotics on leukemia.Front Microbiol. 2024 Jul 3;15:1423838. doi: 10.3389/fmicb.2024.1423838. eCollection 2024. Front Microbiol. 2024. PMID: 39021626 Free PMC article. Review.
-
Emerging CAR immunotherapies: broadening therapeutic horizons beyond cancer.Clin Exp Med. 2025 Aug 4;25(1):274. doi: 10.1007/s10238-025-01820-x. Clin Exp Med. 2025. PMID: 40758198 Free PMC article. Review.
-
Modulating Glycolysis to Improve Cancer Therapy.Int J Mol Sci. 2023 Jan 30;24(3):2606. doi: 10.3390/ijms24032606. Int J Mol Sci. 2023. PMID: 36768924 Free PMC article. Review.
-
Advances in Gene Therapy with Oncolytic Viruses and CAR-T Cells and Therapy-Related Groups.Curr Issues Mol Biol. 2025 Apr 10;47(4):268. doi: 10.3390/cimb47040268. Curr Issues Mol Biol. 2025. PMID: 40699667 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

