Foot-and-mouth disease virus non-structural protein 2B downregulates the RLR signaling pathway via degradation of RIG-I and MDA5
- PMID: 36248821
- PMCID: PMC9556895
- DOI: 10.3389/fimmu.2022.1020262
Foot-and-mouth disease virus non-structural protein 2B downregulates the RLR signaling pathway via degradation of RIG-I and MDA5
Abstract
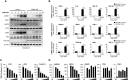
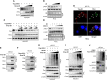
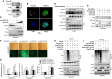
Foot-and-mouth disease virus (FMDV) is a single-stranded, positive-sense RNA virus containing at least 13 proteins. Many of these proteins show immune modulation capabilities. As a non-structural protein of the FMDV, 2B is involved in the rearrangement of the host cell membranes and the disruption of the host secretory pathway as a viroporin. Previous studies have also shown that FMDV 2B plays a role in the modulation of host type-I interferon (IFN) responses through the inhibition of expression of RIG-I and MDA5, key cytosolic sensors of the type-I IFN signaling. However, the exact molecular mechanism is poorly understood. Here, we demonstrated that FMDV 2B modulates host IFN signal pathway by the degradation of RIG-I and MDA5. FMDV 2B targeted the RIG-I for ubiquitination and proteasomal degradation by recruiting E3 ubiquitin ligase ring finger protein 125 (RNF125) and also targeted MDA5 for apoptosis-induced caspase-3- and caspase-8-dependent degradation. Ultimately, FMDV 2B significantly inhibited RNA virus-induced IFN-β production. Importantly, we identified that the C-terminal amino acids 126-154 of FMDV 2B are essential for 2B-mediated degradation of the RIG-I and MDA5. Collectively, these results provide a clearer understanding of the specific molecular mechanisms used by FMDV 2B to inhibit the IFN responses and a rational approach to virus attenuation for future vaccine development.
Keywords: 2B; MDA5; RIG-I; RNF125; foot and mouth disease virus (FMDV).
Copyright © 2022 Weerawardhana, Uddin, Choi, Pathinayake, Shin, Chathuranga, Park and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Foot-and-mouth disease virus non-structural protein 2B negatively regulates the RLR-mediated IFN-β induction.Biochem Biophys Res Commun. 2018 Sep 26;504(1):238-244. doi: 10.1016/j.bbrc.2018.08.161. Epub 2018 Aug 31. Biochem Biophys Res Commun. 2018. PMID: 30177393
-
Foot-and-Mouth Disease Virus 3C Protease Antagonizes Interferon Signaling and C142T Substitution Attenuates the FMD Virus.Front Microbiol. 2021 Nov 19;12:737031. doi: 10.3389/fmicb.2021.737031. eCollection 2021. Front Microbiol. 2021. PMID: 34867853 Free PMC article.
-
Foot-and-Mouth Disease Virus Viroporin 2B Antagonizes RIG-I-Mediated Antiviral Effects by Inhibition of Its Protein Expression.J Virol. 2016 Nov 28;90(24):11106-11121. doi: 10.1128/JVI.01310-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27707918 Free PMC article.
-
Recent Advances and Contradictions in the Study of the Individual Roles of Ubiquitin Ligases That Regulate RIG-I-Like Receptor-Mediated Antiviral Innate Immune Responses.Front Immunol. 2020 Jun 24;11:1296. doi: 10.3389/fimmu.2020.01296. eCollection 2020. Front Immunol. 2020. PMID: 32670286 Free PMC article. Review.
-
Accessory Factors of Cytoplasmic Viral RNA Sensors Required for Antiviral Innate Immune Response.Front Immunol. 2016 May 25;7:200. doi: 10.3389/fimmu.2016.00200. eCollection 2016. Front Immunol. 2016. PMID: 27252702 Free PMC article. Review.
Cited by
-
CD97 negatively regulates the innate immune response against RNA viruses by promoting RNF125-mediated RIG-I degradation.Cell Mol Immunol. 2023 Dec;20(12):1457-1471. doi: 10.1038/s41423-023-01103-z. Epub 2023 Nov 17. Cell Mol Immunol. 2023. PMID: 37978243 Free PMC article.
-
Comparative Transcriptomics Analysis of Foot-and-Mouth Disease Virus-Infected Cell Model Systems.Vet Sci. 2025 Feb 1;12(2):107. doi: 10.3390/vetsci12020107. Vet Sci. 2025. PMID: 40005867 Free PMC article.
-
Virulence and Immune Evasion Strategies of FMDV: Implications for Vaccine Design.Vaccines (Basel). 2024 Sep 19;12(9):1071. doi: 10.3390/vaccines12091071. Vaccines (Basel). 2024. PMID: 39340101 Free PMC article. Review.
-
Use of virus-like particles and nanoparticle-based vaccines for combating picornavirus infections.Vet Res. 2024 Sep 30;55(1):128. doi: 10.1186/s13567-024-01383-x. Vet Res. 2024. PMID: 39350170 Free PMC article. Review.
-
Foot-and-Mouth Disease Virus Capsid Protein VP1 Antagonizes Type I Interferon Signaling via Degradation of Histone Deacetylase 5.Cells. 2024 Mar 19;13(6):539. doi: 10.3390/cells13060539. Cells. 2024. PMID: 38534383 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

