Modulation of urelumab glycosylation separates immune stimulatory activity from organ toxicity
- PMID: 36248847
- PMCID: PMC9558126
- DOI: 10.3389/fimmu.2022.970290
Modulation of urelumab glycosylation separates immune stimulatory activity from organ toxicity
Abstract
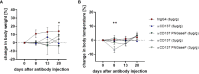
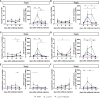
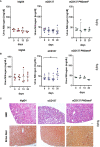
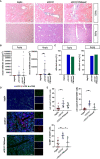
Checkpoint control and immunomodulatory antibodies have become important tools for modulating tumor or self-reactive immune responses. A major issue preventing to make full use of the potential of these immunomodulatory antibodies are the severe side-effects, ranging from systemic cytokine release syndrome to organ-specific toxicities. The IgG Fc-portion has been demonstrated to contribute to both, the desired as well as the undesired antibody activities of checkpoint control and immunomodulatory antibodies via binding to cellular Fcγ-receptors (FcγR). Thus, choosing IgG subclasses, such as human IgG4, with a low ability to interact with FcγRs has been identified as a potential strategy to limit FcγR or complement pathway dependent side-effects. However, even immunomodulatory antibodies on the human IgG4 background may interact with cellular FcγRs and show dose limiting toxicities. By using a humanized mouse model allowing to study the immunomodulatory activity of human checkpoint control antibodies in vivo, we demonstrate that deglycosylation of the CD137-specific IgG4 antibody urelumab results in an amelioration of liver toxicity, while maintaining T cell stimulatory activity. In addition, our results emphasize that antibody dosing impacts the separation of side-effects of urelumab from its therapeutic activity via IgG deglycosylation. Thus, glycoengineering of human IgG4 antibodies may be a possible approach to limit collateral damage by immunomodulatory antibodies and allow for a greater therapeutic window of opportunity.
Keywords: CD137; Fc-receptors; glycosylation; therapeutic antibody; urelumab.
Copyright © 2022 Reitinger, Ipsen-Escobedo, Hornung, Heger, Dudziak, Lux and Nimmerjahn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
FcγR requirements and costimulatory capacity of Urelumab, Utomilumab, and Varlilumab.Front Immunol. 2023 Jul 27;14:1208631. doi: 10.3389/fimmu.2023.1208631. eCollection 2023. Front Immunol. 2023. PMID: 37575254 Free PMC article.
-
Potential of Murine IgG1 and Human IgG4 to Inhibit the Classical Complement and Fcγ Receptor Activation Pathways.Front Immunol. 2018 May 9;9:958. doi: 10.3389/fimmu.2018.00958. eCollection 2018. Front Immunol. 2018. PMID: 29867943 Free PMC article.
-
An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations.Methods. 2014 Jan 1;65(1):114-26. doi: 10.1016/j.ymeth.2013.06.035. Epub 2013 Jul 17. Methods. 2014. PMID: 23872058
-
The role of differential IgG glycosylation in the interaction of antibodies with FcγRs in vivo.Curr Opin Organ Transplant. 2011 Feb;16(1):7-14. doi: 10.1097/MOT.0b013e328342538f. Curr Opin Organ Transplant. 2011. PMID: 21150612 Review.
-
Functional diversification of IgGs through Fc glycosylation.J Clin Invest. 2019 Sep 3;129(9):3492-3498. doi: 10.1172/JCI130029. J Clin Invest. 2019. PMID: 31478910 Free PMC article. Review.
Cited by
-
Delivering co-stimulatory tumor necrosis factor receptor agonism for cancer immunotherapy: past, current and future perspectives.Front Immunol. 2023 Apr 25;14:1147467. doi: 10.3389/fimmu.2023.1147467. eCollection 2023. Front Immunol. 2023. PMID: 37180119 Free PMC article. Review.
-
Agonism of 4-1BB for immune therapy: a perspective on possibilities and complications.Front Immunol. 2023 Aug 17;14:1228486. doi: 10.3389/fimmu.2023.1228486. eCollection 2023. Front Immunol. 2023. PMID: 37662949 Free PMC article. Review.
-
Fcγ-Receptor-Independent Controlled Activation of CD40 Canonical Signaling by Novel Therapeutic Antibodies for Cancer Therapy.Antibodies (Basel). 2024 Apr 18;13(2):31. doi: 10.3390/antib13020031. Antibodies (Basel). 2024. PMID: 38651411 Free PMC article.
-
Fibroblasts: invigorated targets in pre-metastatic niche formation.Int J Biol Sci. 2024 Jan 21;20(3):1110-1124. doi: 10.7150/ijbs.87680. eCollection 2024. Int J Biol Sci. 2024. PMID: 38322116 Free PMC article. Review.
-
Effect of posttranslational modifications and subclass on IgG activity: from immunity to immunotherapy.Nat Immunol. 2023 Aug;24(8):1244-1255. doi: 10.1038/s41590-023-01544-8. Epub 2023 Jul 6. Nat Immunol. 2023. PMID: 37414906 Review.
References
-
- Sun Y, Chen JH, Fu Y. Immunotherapy with agonistic anti-CD137: two sides of a coin. Cell Mol Immunol (2004) 1:31–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

