The role of the astrocyte in subarachnoid hemorrhage and its therapeutic implications
- PMID: 36248855
- PMCID: PMC9556431
- DOI: 10.3389/fimmu.2022.1008795
The role of the astrocyte in subarachnoid hemorrhage and its therapeutic implications
Abstract
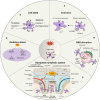
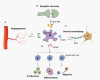
Subarachnoid hemorrhage (SAH) is an important public health concern with high morbidity and mortality worldwide. SAH induces cell death, blood-brain barrier (BBB) damage, brain edema and oxidative stress. As the most abundant cell type in the central nervous system, astrocytes play an essential role in brain damage and recovery following SAH. This review describes astrocyte activation and polarization after SAH. Astrocytes mediate BBB disruption, glymphatic-lymphatic system dysfunction, oxidative stress, and cell death after SAH. Furthermore, astrocytes engage in abundant crosstalk with other brain cells, such as endothelial cells, neurons, pericytes, microglia and monocytes, after SAH. In addition, astrocytes also exert protective functions in SAH. Finally, we summarize evidence regarding therapeutic approaches aimed at modulating astrocyte function following SAH, which could provide some new leads for future translational therapy to alleviate damage after SAH.
Keywords: astrocyte; astrocyte activation; blood-brain barrier; cell death; glymphatic-meningeal lymphatic system; neurovascular unit; oxidative stress; subarachnoid hemorrhage.
Copyright © 2022 Li, Zhao, Yao, Zhou, Lenahan, Wang, Ou and He.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
PEDF-34 attenuates neurological deficit and suppresses astrocyte-dependent neuroinflammation by modulating astrocyte polarization via 67LR/JNK/STAT1 signaling pathway after subarachnoid hemorrhage in rats.J Neuroinflammation. 2024 Jul 21;21(1):178. doi: 10.1186/s12974-024-03171-y. J Neuroinflammation. 2024. PMID: 39034417 Free PMC article.
-
Astrocyte-microglia crosstalk in subarachnoid hemorrhage: mechanisms and treatments.Front Immunol. 2025 Jun 30;16:1547858. doi: 10.3389/fimmu.2025.1547858. eCollection 2025. Front Immunol. 2025. PMID: 40661949 Free PMC article. Review.
-
The blood-brain barrier and the neurovascular unit in subarachnoid hemorrhage: molecular events and potential treatments.Fluids Barriers CNS. 2022 Apr 11;19(1):29. doi: 10.1186/s12987-022-00312-4. Fluids Barriers CNS. 2022. PMID: 35410231 Free PMC article. Review.
-
EphA4/EphrinB2 signaling mediates pericyte-induced transient glia limitans formation as a secondary protective barrier after subarachnoid hemorrhage in mice.Exp Neurol. 2023 Feb;360:114293. doi: 10.1016/j.expneurol.2022.114293. Epub 2022 Dec 6. Exp Neurol. 2023. PMID: 36493862 Free PMC article.
-
Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats.Stroke. 2010 Aug;41(8):1783-90. doi: 10.1161/STROKEAHA.110.586537. Epub 2010 Jul 8. Stroke. 2010. PMID: 20616319 Free PMC article.
Cited by
-
Promise of mesenchymal stem cell-derived extracellular vesicles for alleviating subarachnoid hemorrhage-induced brain dysfunction by neuroprotective and antiinflammatory effects.Brain Behav Immun Health. 2024 Aug 3;40:100835. doi: 10.1016/j.bbih.2024.100835. eCollection 2024 Oct. Brain Behav Immun Health. 2024. PMID: 39165307 Free PMC article. Review.
-
Role of GPX3+ astrocytes in breast cancer brain metastasis activated by circulating tumor cell exosomes.NPJ Precis Oncol. 2025 Mar 7;9(1):64. doi: 10.1038/s41698-025-00833-9. NPJ Precis Oncol. 2025. PMID: 40055530 Free PMC article.
-
Cortical Spreading Depolarization and Delayed Cerebral Ischemia; Rethinking Secondary Neurological Injury in Subarachnoid Hemorrhage.Int J Mol Sci. 2023 Jun 8;24(12):9883. doi: 10.3390/ijms24129883. Int J Mol Sci. 2023. PMID: 37373029 Free PMC article. Review.
-
Biomarkers in Aneurysmatic and Spontaneous Subarachnoid Haemorrhage: A Clinical Prospective Multicentre Biomarker Panel Study of S100B, Claudin-5, Interleukin-10, TREM-1, TREM-2 and Neurofilament Light Chain As Well As Immunoglobulin G and M.Mol Neurobiol. 2025 Sep;62(9):11499-11516. doi: 10.1007/s12035-025-04889-3. Epub 2025 Apr 28. Mol Neurobiol. 2025. PMID: 40295361 Free PMC article.
-
Development and Validation of a Prechiasmatic Mouse Model of Subarachnoid Hemorrhage to Measure Long-Term Cognitive Deficits.Adv Sci (Weinh). 2024 Dec;11(46):e2403977. doi: 10.1002/advs.202403977. Epub 2024 Oct 23. Adv Sci (Weinh). 2024. PMID: 39443821 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

