Combination treatment of radiofrequency ablation and peptide neoantigen vaccination: Promising modality for future cancer immunotherapy
- PMID: 36248865
- PMCID: PMC9559398
- DOI: 10.3389/fimmu.2022.1000681
Combination treatment of radiofrequency ablation and peptide neoantigen vaccination: Promising modality for future cancer immunotherapy
Abstract
Background: The safety and immunogenicity of a personalized neoantigen-based peptide vaccine, iNeo-Vac-P01, was reported previously in patients with a variety of cancer types. The current study investigated the synergistic effects of radiofrequency ablation (RFA) and neoantigen vaccination in cancer patients and tumor-bearing mice.
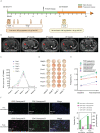
Methods: Twenty-eight cancer patients were enrolled in this study, including 10 patients who had received RFA treatment within 6 months before vaccination (Cohort 1), and 18 patients who had not (Cohort 2). Individualized neoantigen peptide vaccines were designed, manufactured, and subcutaneously administrated with GM-CSF as an adjuvant for all patients. Mouse models were employed to validate the synergistic efficacy of combination treatment of RFA and neoantigen vaccination.
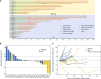
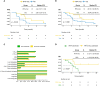
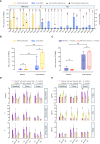
Results: Longer median progression free survival (mPFS) and median overall survival (mOS) were observed in patients in Cohort 1 compared to patients in Cohort 2 (4.42 and 20.18 months vs. 2.82 and 10.94 months). The results of ex vivo IFN-γ ELISpot assay showed that patients in Cohort 1 had stronger neoantigen-specific immune responses at baseline and post vaccination. Mice receiving combination treatment of RFA and neoantigen vaccines displayed higher antitumor immune responses than mice receiving single modality. The combination of PD-1 blockage with RFA and neoantigen vaccines further enhanced the antitumor response in mice.
Conclusion: Neoantigen vaccination after local RFA treatment could improve the clinical and immune response among patients of different cancer types. The synergistic antitumor potentials of these two modalities were also validated in mice, and might be further enhanced by immune checkpoint inhibition. The mechanisms of their synergies require further investigation.
Clinical trial registration: https://clinicaltrials.gov/, identifier NCT03662815.
Keywords: cancer; immune checkpoint inhibition; immunotherapy; neoantigen vaccine; radiofrequency ablation.
Copyright © 2022 Shou, Mo, Zhang, Lu, Han, Liu, Qiu, Li, Han, Ma, Guo, Guo, Huang, Zhang, Ye, Pan, Chen and Fang.
Conflict of interest statement
FM, SZ, LLu, NH, LLiu, MQ, DM, XG, QH, XZ, and SC are employed by Hangzhou Neoantigen Therapeutics Co., Ltd. FM is employed by company Hangzhou AI-Force Therapeutics Co., Ltd. NH is employed by company Hangzhou AI-Nano Therapeutics Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
A Neoantigen-Based Peptide Vaccine for Patients With Advanced Pancreatic Cancer Refractory to Standard Treatment.Front Immunol. 2021 Aug 13;12:691605. doi: 10.3389/fimmu.2021.691605. eCollection 2021. Front Immunol. 2021. PMID: 34484187 Free PMC article.
-
A Pan-cancer Clinical Study of Personalized Neoantigen Vaccine Monotherapy in Treating Patients with Various Types of Advanced Solid Tumors.Clin Cancer Res. 2020 Sep 1;26(17):4511-4520. doi: 10.1158/1078-0432.CCR-19-2881. Epub 2020 May 21. Clin Cancer Res. 2020. PMID: 32439700
-
Personalized neoantigen-pulsed dendritic cell vaccines show superior immunogenicity to neoantigen-adjuvant vaccines in mouse tumor models.Cancer Immunol Immunother. 2020 Jan;69(1):135-145. doi: 10.1007/s00262-019-02448-z. Epub 2019 Dec 5. Cancer Immunol Immunother. 2020. PMID: 31807878 Free PMC article.
-
Advances in personalized neoantigen vaccines for cancer immunotherapy.Biosci Trends. 2020 Nov 4;14(5):349-353. doi: 10.5582/bst.2020.03267. Epub 2020 Sep 10. Biosci Trends. 2020. PMID: 32908077 Review.
-
Radiofrequency ablation of liver metastasis: potential impact on immune checkpoint inhibitor therapy.Eur Radiol. 2019 Sep;29(9):5045-5051. doi: 10.1007/s00330-019-06189-6. Epub 2019 Apr 8. Eur Radiol. 2019. PMID: 30963271 Review.
Cited by
-
Personalized Cancer Vaccines Go Viral: Viral Vectors in the Era of Personalized Immunotherapy of Cancer.Int J Mol Sci. 2023 Nov 22;24(23):16591. doi: 10.3390/ijms242316591. Int J Mol Sci. 2023. PMID: 38068911 Free PMC article. Review.
-
Harnessing endoscopic ultrasound-guided radiofrequency ablation to reshape the pancreatic ductal adenocarcinoma microenvironment and elicit systemic immunomodulation.Explor Target Antitumor Ther. 2024;5(5):1056-1073. doi: 10.37349/etat.2024.00263. Epub 2024 Aug 15. Explor Target Antitumor Ther. 2024. PMID: 39351436 Free PMC article. Review.
-
The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells.Mol Cancer. 2023 Aug 30;22(1):141. doi: 10.1186/s12943-023-01844-5. Mol Cancer. 2023. PMID: 37649123 Free PMC article. Review.
-
Transformers meets neoantigen detection: a systematic literature review.J Integr Bioinform. 2024 Jul 4;21(2):20230043. doi: 10.1515/jib-2023-0043. eCollection 2024 Jun 1. J Integr Bioinform. 2024. PMID: 38960869 Free PMC article.
-
Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment.J Funct Biomater. 2024 Aug 16;15(8):229. doi: 10.3390/jfb15080229. J Funct Biomater. 2024. PMID: 39194667 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

