Peripheral blood marker of residual acute leukemia after hematopoietic cell transplantation using multi-plex digital droplet PCR
- PMID: 36248870
- PMCID: PMC9556966
- DOI: 10.3389/fimmu.2022.999298
Peripheral blood marker of residual acute leukemia after hematopoietic cell transplantation using multi-plex digital droplet PCR
Abstract
Background: Relapse remains the primary cause of death after hematopoietic cell transplantation (HCT) for acute leukemia. The ability to identify minimal/measurable residual disease (MRD) via the blood could identify patients earlier when immunologic interventions may be more successful. We evaluated a new test that could quantify blood tumor mRNA as leukemia MRD surveillance using droplet digital PCR (ddPCR).
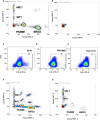
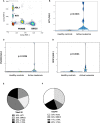
Methods: The multiplex ddPCR assay was developed using tumor cell lines positive for the tumor associated antigens (TAA: WT1, PRAME, BIRC5), with homeostatic ABL1. On IRB-approved protocols, RNA was isolated from mononuclear cells from acute leukemia patients after HCT (n = 31 subjects; n = 91 specimens) and healthy donors (n = 20). ddPCR simultaneously quantitated mRNA expression of WT1, PRAME, BIRC5, and ABL1 and the TAA/ABL1 blood ratio was measured in patients with and without active leukemia after HCT.
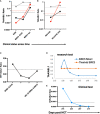
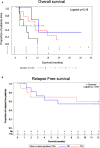
Results: Tumor cell lines confirmed quantitation of TAAs. In patients with active acute leukemia after HCT (MRD+ or relapse; n=19), the blood levels of WT1/ABL1, PRAME/ABL1, and BIRC5/ABL1 exceeded healthy donors (p<0.0001, p=0.0286, and p=0.0064 respectively). Active disease status was associated with TAA positivity (1+ TAA vs 0 TAA) with an odds ratio=10.67, (p=0.0070, 95% confidence interval 1.91 - 59.62). The area under the curve is 0.7544. Changes in ddPCR correlated with disease response captured on standard of care tests, accurately denoting positive or negative disease burden in 15/16 (95%). Of patients with MRD+ or relapsed leukemia after HCT, 84% were positive for at least one TAA/ABL1 in the peripheral blood. In summary, we have developed a new method for blood MRD monitoring of leukemia after HCT and present preliminary data that the TAA/ABL1 ratio may may serve as a novel surrogate biomarker for relapse of acute leukemia after HCT.
Keywords: acute leukemia; digital droplet PCR; hematopoietic cell transplantation; minimal residual disease; relapse.
Copyright © 2022 Stanojevic, Grant, Vesely, Knoblach, Kanakry, Nazarian, Panditharatna, Panchapakesan, Gress, Holter-Chakrabarty and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Zuanelli Brambilla C, Lobaugh SM, Ruiz JD, Dahi PB, Goldberg AD, Young JW, et al. . Relapse after allogeneic stem cell transplantation of acute myelogenous leukemia and myelodysplastic syndrome and the importance of second cellular therapy. Transplant Cell Ther (2021) 27(9):771 e1– e10. doi: 10.1016/j.jtct.2021.05.011 - DOI - PMC - PubMed
-
- Styczynski J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. . Death after hematopoietic stem cell transplantation: Changes over calendar year time, infections and associated factors. Bone marrow Transplant (2020) 55(1):126–36. doi: 10.1038/s41409-019-0624-z - DOI - PMC - PubMed
-
- Bastos-Oreiro M, Perez-Corral A, Martinez-Laperche C, Bento L, Pascual C, Kwon M, et al. . Prognostic impact of minimal residual disease analysis by flow cytometry in patients with acute myeloid leukemia before and after allogeneic hemopoietic stem cell transplantation. Eur J haematol (2014) 93(3):239–46. doi: 10.1111/ejh.12336 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

