Pyroptosis and respiratory diseases: A review of current knowledge
- PMID: 36248872
- PMCID: PMC9561627
- DOI: 10.3389/fimmu.2022.920464
Pyroptosis and respiratory diseases: A review of current knowledge
Abstract
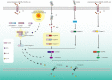
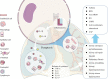
Pyroptosis is a relatively newly discovered programmed cell death accompanied by an inflammatory response. In the classical view, pyroptosis is mediated by caspases-1,-4,-5,-11 and executed by GSDMD, however, recently it was demonstrated that caspase-3 and-8 also participate in the process of pyroptosis, by cleaving GSDMD/E and GSDMD respectively. Different from autophagy and apoptosis, many pores are formed on the cell membrane during pyroptosis, which makes the cell membrane lose its integrity, eventually leading to the release of cytokines interleukin(IL)-1β and IL-18. When the body is infected with pathogens or exposed to some stimulations, pyroptosis could play an immune defense role. It is found that pyroptosis exists widely in infectious and inflammatory respiratory diseases such as acute lung injury, bronchial dysplasia, chronic obstructive pulmonary disease, and asthma. Excessive pyroptosis may accompany airway inflammation, tissue injury, and airway damage, and induce an inflammatory reaction, leading to more serious damage and poor prognosis of respiratory diseases. This review summarizes the relationship between pyroptosis and related respiratory diseases.
Keywords: NLRP3; caspase; infection; inflammation; pyroptosis; respiratory diseases.
Copyright © 2022 Sun and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Gasdermin D is involved in switching from apoptosis to pyroptosis in TLR4-mediated renal tubular epithelial cells injury in diabetic kidney disease.Arch Biochem Biophys. 2022 Sep 30;727:109347. doi: 10.1016/j.abb.2022.109347. Epub 2022 Jul 7. Arch Biochem Biophys. 2022. PMID: 35809639
-
Mechanism and Regulation of Gasdermin-Mediated Cell Death.Cold Spring Harb Perspect Biol. 2020 Mar 2;12(3):a036400. doi: 10.1101/cshperspect.a036400. Cold Spring Harb Perspect Biol. 2020. PMID: 31451512 Free PMC article. Review.
-
Wedelolactone ameliorates Pseudomonas aeruginosa-induced inflammation and corneal injury by suppressing caspase-4/5/11/GSDMD-mediated non-canonical pyroptosis.Exp Eye Res. 2021 Oct;211:108750. doi: 10.1016/j.exer.2021.108750. Epub 2021 Sep 2. Exp Eye Res. 2021. PMID: 34481822
-
Suppression of the caspase-1/GSDMD-mediated pyroptotic signaling pathway through dexamethasone alleviates corneal alkali injuries.Exp Eye Res. 2022 Jan;214:108858. doi: 10.1016/j.exer.2021.108858. Epub 2021 Nov 23. Exp Eye Res. 2022. PMID: 34822855
-
Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases.J Cell Physiol. 2020 Apr;235(4):3207-3221. doi: 10.1002/jcp.29268. Epub 2019 Oct 17. J Cell Physiol. 2020. PMID: 31621910 Review.
Cited by
-
Ticagrelor alleviates pyroptosis of myocardial ischemia reperfusion-induced acute lung injury in rats: a preliminary study.PeerJ. 2024 Jan 4;12:e16613. doi: 10.7717/peerj.16613. eCollection 2024. PeerJ. 2024. PMID: 38188139 Free PMC article.
-
Dectin-1 aggravates neutrophil inflammation through caspase-11/4-mediated macrophage pyroptosis in asthma.Respir Res. 2024 Mar 8;25(1):119. doi: 10.1186/s12931-024-02743-z. Respir Res. 2024. PMID: 38459541 Free PMC article.
-
The NLRP3 inflammasome in allergic diseases: mechanisms and therapeutic implications.Clin Exp Med. 2024 Sep 26;24(1):231. doi: 10.1007/s10238-024-01492-z. Clin Exp Med. 2024. PMID: 39325206 Free PMC article. Review.
-
Forms of Non-Apoptotic Cell Death and Their Role in Gliomas-Presentation of the Current State of Knowledge.Biomedicines. 2024 Jul 11;12(7):1546. doi: 10.3390/biomedicines12071546. Biomedicines. 2024. PMID: 39062119 Free PMC article. Review.
-
Acute lung injury: a view from the perspective of necroptosis.Inflamm Res. 2024 Jun;73(6):997-1018. doi: 10.1007/s00011-024-01879-4. Epub 2024 Apr 14. Inflamm Res. 2024. PMID: 38615296 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

