Safety and efficacy of fecal microbiota transplantation for autoimmune diseases and autoinflammatory diseases: A systematic review and meta-analysis
- PMID: 36248877
- PMCID: PMC9562921
- DOI: 10.3389/fimmu.2022.944387
Safety and efficacy of fecal microbiota transplantation for autoimmune diseases and autoinflammatory diseases: A systematic review and meta-analysis
Abstract
Objective: To evaluate the safety and efficacy of fecal microbiota transplantation for autoimmune diseases and autoinflammatory diseases.
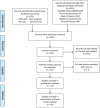
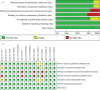
Methods: Relevant literature was retrieved from the PubMed database, Embase database, Cochrane Library database, etc. The search period is from the establishment of the database to January 2022. The outcomes include clinical symptoms, improvement in biochemistry, improvement in intestinal microbiota, improvement in the immune system, and adverse events. Literature screening and data extraction were independently carried out by two researchers according to the inclusion and exclusion criteria, and RevMan 5.3 software was used for statistics and analysis.
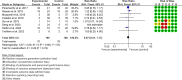
Results: Overall, a total of 14 randomized controlled trials (RCTs) involving six types of autoimmune diseases were included. The results showed the following. 1) Type 1 diabetes mellitus (T1DM): compared with the autologous fecal microbiota transplantation (FMT) group (control group), the fasting plasma C peptide in the allogenic FMT group at 12 months was lower. 2) Systemic sclerosis: at week 4, compared with one of two placebo controls, three patients in the experimental group reported a major improvement in fecal incontinence. 3) Ulcerative colitis, pediatric ulcerative colitis, and Crohn's disease: FMT may increase clinical remission, clinical response, and endoscopic remission for patients with ulcerative colitis and increase clinical remission for patients with Crohn's disease. 4) Psoriatic arthritis: there was no difference in the ratio of ACR20 between the two groups.
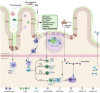
Conclusion: Based on current evidence, the application of FMT in the treatment of autoimmune diseases is effective and relatively safe, and it is expected to be used as a method to induce remission of active autoimmune diseases.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021235055, identifier CRD42021235055.
Keywords: autoimmune diseases; autoinflammatory diseases; fecal microbiota transplantation; meta-analysis; systemic review.
Copyright © 2022 Zeng, Deng, Yang, Chen, He and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
-
Efficacy of Fecal Microbiota Transplantation in the Treatment of Active Ulcerative Colitis: A Systematic Review and Meta-Analysis of Double-Blind Randomized Controlled Trials.Inflamm Bowel Dis. 2023 May 2;29(5):808-817. doi: 10.1093/ibd/izac135. Inflamm Bowel Dis. 2023. PMID: 35766805
-
[Fecal microbiota transplantation for the treatment of ulcerative colitis: a Meta-analysis].Zhonghua Nei Ke Za Zhi. 2019 Mar 1;58(3):202-208. doi: 10.3760/cma.j.issn.0578-1426.2019.03.010. Zhonghua Nei Ke Za Zhi. 2019. PMID: 30803179 Chinese.
-
Assessing the efficacy and safety of fecal microbiota transplantation and probiotic VSL#3 for active ulcerative colitis: A systematic review and meta-analysis.PLoS One. 2020 Mar 17;15(3):e0228846. doi: 10.1371/journal.pone.0228846. eCollection 2020. PLoS One. 2020. PMID: 32182248 Free PMC article.
-
Fecal microbiota transplantation therapy in Crohn's disease: Systematic review.J Gastroenterol Hepatol. 2021 Oct;36(10):2672-2686. doi: 10.1111/jgh.15598. Epub 2021 Jul 6. J Gastroenterol Hepatol. 2021. PMID: 34169565
Cited by
-
Unveiling the hidden world of gut health: Exploring cutting-edge research through visualizing randomized controlled trials on the gut microbiota.World J Clin Cases. 2023 Sep 16;11(26):6132-6146. doi: 10.12998/wjcc.v11.i26.6132. World J Clin Cases. 2023. PMID: 37731574 Free PMC article.
-
Exploring the immunomodulatory mechanism of total glucosides of paeony on Sjögren's syndrome dry eye disease based on the "gut-eye axis" pathway.Int Ophthalmol. 2025 Jun 5;45(1):230. doi: 10.1007/s10792-025-03607-1. Int Ophthalmol. 2025. PMID: 40471444
-
Advances in the study of artemisinin and its derivatives for the treatment of rheumatic skeletal disorders, autoimmune inflammatory diseases, and autoimmune disorders: a comprehensive review.Front Immunol. 2024 Oct 25;15:1432625. doi: 10.3389/fimmu.2024.1432625. eCollection 2024. Front Immunol. 2024. PMID: 39524446 Free PMC article. Review.
-
Bringing gut microbiota into the spotlight of clinical research and medical practice.World J Clin Cases. 2024 May 16;12(14):2293-2300. doi: 10.12998/wjcc.v12.i14.2293. World J Clin Cases. 2024. PMID: 38765739 Free PMC article.
-
Possible Applications of Fecal Microbiota Transplantation in the Pediatric Population: A Systematic Review.Biomedicines. 2025 Jun 6;13(6):1393. doi: 10.3390/biomedicines13061393. Biomedicines. 2025. PMID: 40564111 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

