miR-aculous new avenues for cancer immunotherapy
- PMID: 36248881
- PMCID: PMC9554277
- DOI: 10.3389/fimmu.2022.929677
miR-aculous new avenues for cancer immunotherapy
Abstract
The rising toll of cancer globally necessitates ingenuity in early detection and therapy. In the last decade, the utilization of immune signatures and immune-based therapies has made significant progress in the clinic; however, clinical standards leave many current and future patients without options. Non-coding RNAs, specifically microRNAs, have been explored in pre-clinical contexts with tremendous success. MicroRNAs play indispensable roles in programming the interactions between immune and cancer cells, many of which are current or potential immunotherapy targets. MicroRNAs mechanistically control a network of target genes that can alter immune and cancer cell biology. These insights provide us with opportunities and tools that may complement and improve immunotherapies. In this review, we discuss immune and cancer cell-derived miRNAs that regulate cancer immunity and examine miRNAs as an integral part of cancer diagnosis, classification, and therapy.
Keywords: TME (tumor microenvironment); cancer cell; cancer immunity and immunotherapy; cell death; exosomes; metabolism; microRNA.
Copyright © 2022 Tang, Bauer, Barba, Ekiz and O’Connell.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



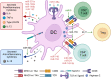
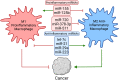
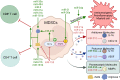
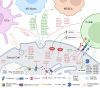
References
-
- Dagher R, Cohen M, Williams G, Rothmann M, Gobburu J, Robbie G, et al. . Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res (2002) 8:3034–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

