Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response
- PMID: 36248891
- PMCID: PMC9561420
- DOI: 10.3389/fimmu.2022.1028209
Microbial dysbiosis and childhood asthma development: Integrated role of the airway and gut microbiome, environmental exposures, and host metabolic and immune response
Abstract
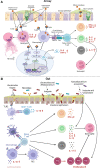
Asthma is a chronic and heterogeneous respiratory disease with many risk factors that typically originate during early childhood. A complex interplay between environmental factors and genetic predisposition is considered to shape the lung and gut microbiome in early life. The growing literature has identified that changes in the relative abundance of microbes (microbial dysbiosis) and reduced microbial diversity, as triggers of the airway-gut axis crosstalk dysregulation, are associated with asthma development. There are several mechanisms underlying microbial dysbiosis to childhood asthma development pathways. For example, a bacterial infection in the airway of infants can lead to the activation and/or dysregulation of inflammatory pathways that contribute to bronchoconstriction and bronchial hyperresponsiveness. In addition, gut microbial dysbiosis in infancy can affect immune development and differentiation, resulting in a suboptimal balance between innate and adaptive immunity. This evolving dysregulation of secretion of pro-inflammatory mediators has been associated with persistent airway inflammation and subsequent asthma development. In this review, we examine current evidence around associations between the airway and gut microbial dysbiosis with childhood asthma development. More specifically, this review focuses on discussing the integrated roles of environmental exposures, host metabolic and immune responses, airway and gut microbial dysbiosis in driving childhood asthma development.
Keywords: airway microbiome; childhood asthma; gut microbiome; immune mechanism; metabolic mechanism; microbial dysbiosis.
Copyright © 2022 Liu, Makrinioti, Saglani, Bowman, Lin, Camargo, Hasegawa and Zhu.
Conflict of interest statement
CL, MB, and L-LL are employees of Sanofi US and may hold shares and/or stock options in the company. CC and KH report grants from National Institutes of Health outside the submitted work. ZZ reports grants from National Institutes of Health and Harvard University during the conduct of the study. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dysbiosis of the gut and lung microbiome has a role in asthma.Semin Immunopathol. 2020 Feb;42(1):75-93. doi: 10.1007/s00281-019-00775-y. Epub 2020 Feb 18. Semin Immunopathol. 2020. PMID: 32072252 Free PMC article. Review.
-
Airway microbial dysbiosis in asthmatic patients: A target for prevention and treatment?J Allergy Clin Immunol. 2017 Apr;139(4):1071-1081. doi: 10.1016/j.jaci.2017.02.004. J Allergy Clin Immunol. 2017. PMID: 28390574 Review.
-
Factors influencing the gut microbiome in children: from infancy to childhood.J Biosci. 2019 Jun;44(2):49. J Biosci. 2019. PMID: 31180062 Review.
-
Dysbiosis of the intestinal fungal microbiota increases lung resident group 2 innate lymphoid cells and is associated with enhanced asthma severity in mice and humans.Respir Res. 2023 May 31;24(1):144. doi: 10.1186/s12931-023-02422-5. Respir Res. 2023. PMID: 37259076 Free PMC article.
-
Alteration of Lung and Gut Microbiota in IL-13-Transgenic Mice Simulating Chronic Asthma.J Microbiol Biotechnol. 2020 Dec 28;30(12):1819-1826. doi: 10.4014/jmb.2009.09019. J Microbiol Biotechnol. 2020. PMID: 33046682 Free PMC article.
Cited by
-
Update on the association between Helicobacter pylori infection and asthma in terms of microbiota and immunity.Allergy Asthma Clin Immunol. 2024 Jan 14;20(1):4. doi: 10.1186/s13223-024-00870-2. Allergy Asthma Clin Immunol. 2024. PMID: 38221621 Free PMC article. Review.
-
The Gut-Organ Axis within the Human Body: Gut Dysbiosis and the Role of Prebiotics.Life (Basel). 2023 Oct 8;13(10):2023. doi: 10.3390/life13102023. Life (Basel). 2023. PMID: 37895405 Free PMC article. Review.
-
Microbiome modifications by steroids during viral exacerbation of asthma and in healthy mice.Am J Physiol Lung Cell Mol Physiol. 2024 Nov 1;327(5):L646-L660. doi: 10.1152/ajplung.00040.2024. Epub 2024 Aug 19. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 39159427
-
Integrated nasopharyngeal airway metagenome and asthma genetic risk endotyping of severe bronchiolitis in infancy and risk of childhood asthma.Eur Respir J. 2024 Sep 26;64(6):2401130. doi: 10.1183/13993003.01130-2024. Print 2024 Dec. Eur Respir J. 2024. PMID: 39326916 Free PMC article.
-
Can probiotics be used in the prevention and treatment of bronchial asthma?Pharmacol Rep. 2024 Aug;76(4):740-753. doi: 10.1007/s43440-024-00618-0. Epub 2024 Jul 1. Pharmacol Rep. 2024. PMID: 38951480 Free PMC article. Review.
References
-
- The Global Initiative for Asthma . 2022 gina report, global strategy for asthma management and prevention. (2022).

