Cross-reactivity between vaccine antigens from the chitin deacetylase protein family improves survival in a mouse model of cryptococcosis
- PMID: 36248898
- PMCID: PMC9554598
- DOI: 10.3389/fimmu.2022.1015586
Cross-reactivity between vaccine antigens from the chitin deacetylase protein family improves survival in a mouse model of cryptococcosis
Abstract
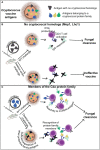
Meningitis due to the fungal pathogen Cryptococcus neoformans is estimated to cause nearly 200,000 deaths annually, mostly in resource-limited regions. We previously identified cryptococcal protein antigens which, when delivered in glucan particles, afford vaccine-mediated protection against an otherwise lethal infection. Many of these proteins exhibit significant homology to other similar cryptococcal proteins leading us to hypothesize that protection may be augmented by immunologic cross-reactivity to multiple members of a protein family. To examine the significance of protein cross-reactivity in vaccination, we utilized strains of Cryptococcus that are genetically deficient in select antigens, yet are still lethal in mice. Vaccination with a protein without homologs (e.g., Mep1 and Lhc1) protected against challenge with wild-type Cryptococcus, but not against a deletion strain lacking that protein. Contrastingly, vaccination with a single chitin deacetylase (Cda) protein protected against the corresponding deletion strain, presumably due to host recognition of one or more other family members still expressed in this strain. Vaccination with a single Cda protein induced cross-reactive antibody and interferon-gamma (IFNγ) immune responses to other Cda protein family members. Paradoxically, we saw no evidence of cross-protection within the carboxypeptidase family of proteins. Factors such as in vivo protein expression and the degree of homology across the family could inform the extent to which vaccine-mediated immunity is amplified. Together, these data suggest a role for prioritizing protein families in fungal vaccine design: increasing the number of immune targets generated by a single antigen may improve efficacy while diminishing the risk of vaccine-resistant strains arising from gene mutations.
Keywords: CD4 T cell; Cryptococcus; acquired immunodeficiency syndrome; cross-protection; fungal vaccine; protein family.
Copyright © 2022 Hester, Oliveira, Wang, Mou, Lourenco, Ostroff, Specht and Levitz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


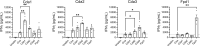


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

