Mechanisms of mutant β-catenin in endometrial cancer progression
- PMID: 36248967
- PMCID: PMC9556987
- DOI: 10.3389/fonc.2022.1009345
Mechanisms of mutant β-catenin in endometrial cancer progression
Abstract
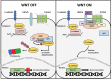
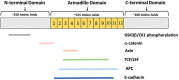
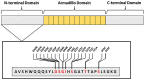
Endometrial carcinoma (EC) is the most diagnosed gynecological malignancy in Western countries. Both incidence and mortality rates of EC have steadily risen in recent years. Despite generally favorable prognoses for patients with the endometrioid type of EC, a subset of patients has been identified with decreased progression-free survival. Patients in this group are distinguished from other endometrioid EC patients by the presence of exon 3 hotspot mutations in CTNNB1, the gene encoding for the β-catenin protein. β-catenin is an evolutionarily conserved protein with critical functions in both adherens junctions and Wnt-signaling. The exact mechanism by which exon 3 CTNNB1 mutations drive EC progression is not well understood. Further, the potential contribution of mutant β-catenin to adherens junctions' integrity is not known. Additionally, the magnitude of worsened progression-free survival in patients with CTNNB1 mutations is context dependent, and therefore the importance of this subset of patients can be obscured by improper categorization. This review will examine the history and functions of β-catenin, how these functions may change and drive EC progression in CTNNB1 mutant patients, and the importance of this patient group in the broader context of the disease.
Keywords: Wnt-signaling; cell adhesion; endometrial cancer; tumor progression; β-catenin.
Copyright © 2022 Parrish, Broaddus and Gladden.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

