Screening the PRISM Library against Staphylococcus aureus Reveals a Sesquiterpene Lactone from Liriodendron tulipifera with Inhibitory Activity
- PMID: 36249352
- PMCID: PMC9558601
- DOI: 10.1021/acsomega.2c03539
Screening the PRISM Library against Staphylococcus aureus Reveals a Sesquiterpene Lactone from Liriodendron tulipifera with Inhibitory Activity
Abstract
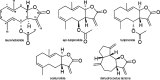
Infections caused by the bacterium Staphylococcus aureus continue to pose threats to human health and put a financial burden on the healthcare system. The overuse of antibiotics has contributed to mutations leading to the emergence of methicillin-resistant S. aureus, and there is a critical need for the discovery and development of new antibiotics to evade drug-resistant bacteria. Medicinal plants have shown promise as sources of new small-molecule therapeutics with potential uses against pathogenic infections. The principal Rhode Island secondary metabolite (PRISM) library is a botanical extract library generated from specimens in the URI Youngken Medicinal Garden by upper-division undergraduate students. PRISM extracts were screened for activity against strains of methicillin-susceptible S. aureus (MSSA). An extract generated from the tulip tree (Liriodendron tulipifera) demonstrated growth inhibition against MSSA, and a bioassay-guided approach identified a sesquiterpene lactone, laurenobiolide, as the active constituent. Intriguingly, its isomers, tulipinolide and epi-tulipinolide, lacked potent activity against MSSA. Laurenobiolide also proved to be more potent against MSSA than the structurally similar sesquiterpene lactones, costunolide and dehydrocostus lactone. Laurenobiolide was the most abundant in the twig bark of the tulip tree, supporting the twig bark's historical and cultural usage in poultices and teas.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
