Interfacial Oxidative Oligomerization of Catechol
- PMID: 36249361
- PMCID: PMC9558612
- DOI: 10.1021/acsomega.2c05290
Interfacial Oxidative Oligomerization of Catechol
Abstract
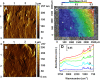
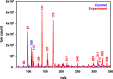
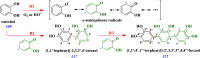
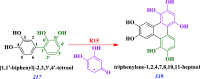
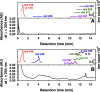
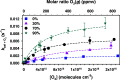
The heterogeneous reaction between thin films of catechol exposed to O3(g) creates hydroxyl radicals (HO•) in situ, which in turn generate semiquinone radical intermediates in the path to form heavier polyhydroxylated biphenyl, terphenyl, and triphenylene products. Herein, the alteration of catechol aromatic surfaces and their chemical composition are studied during the heterogeneous oxidation of catechol films by O3(g) molar ratios ≥ 230 ppbv at variable relative humidity levels (0% ≤ RH ≤ 90%). Fourier transform infrared micro-spectroscopy, atomic force microscopy, electrospray ionization mass spectrometry, and reverse-phase liquid chromatography with UV-visible and mass spectrometry detection provide new physical insights into understanding the surface reaction. A Langmuir-Hinshelwood mechanism is accounted to report reaction rates, half-lives, and reactive uptake coefficients for the system under variable relative humidity levels. The reactions reported explain how the oligomerization of polyphenols proceeds at interfaces to contribute to the formation of brown organic carbon in atmospheric aerosols.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Avdeef A.; Sofen S. R.; Bregante T. L.; Raymond K. N. Coordination chemistry of microbial iron transport compounds. 9. Stability constants for catechol models of enterobactin. J. Am. Chem. Soc. 1978, 100, 5362.10.1021/ja00485a018. - DOI
LinkOut - more resources
Full Text Sources
