Clinical Performance of Rapid Antigen Tests for the Detection of SARS-CoV-2 Infection in the Emergency Department and Community: A Retrospective Study
- PMID: 36249591
- PMCID: PMC9560843
- DOI: 10.1155/2022/9447251
Clinical Performance of Rapid Antigen Tests for the Detection of SARS-CoV-2 Infection in the Emergency Department and Community: A Retrospective Study
Abstract
Background: Rapid antigen tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection have been authorized for emergency use (EUA); however, the performance has not been fully evaluated in clinical contexts. This study aimed to provide evidence regarding the diagnostic performance of SARS-CoV-2 rapid antigen tests compared with the real-time reverse transcription-polymerase chain reaction (RT-PCR) test in the emergency department (ED) and community.
Methods: Patients who underwent SARS-CoV-2 rapid antigen tests using the VTRUST COVID-19 Antigen Rapid Test (TD-4531) and real-time RT-PCR on the same day in the ED or community from May 24, 2021, to June 24, 2021, were examined.
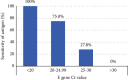
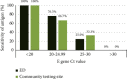
Results: Paired nasopharyngeal swabs were collected from 4022 suspected COVID-19 patients: 800 in the ED and 3222 in the community. Overall, 62 (1.54%) tested positive, 13 tested indeterminate, and 3947 tested negative by real-time RT-PCR. The sensitivity and specificity of the antigen test were 51.61% and 99.44% (overall), 62.50% and 99.61% (ED), and 31.82% and 99.40% (community), respectively. There were 30 false negatives and 22 false positives. Among the false negatives, 16.67% had a cycle threshold (Ct) value of <25.
Conclusion: The VTRUST COVID-19 Antigen Rapid Test showed comparable specificity as real-time RT-PCR for the ED and community, but the sensitivity was relatively low, especially when the Ct value was >25. This test can be useful for the rapid identification of infected subjects in an epidemic situation.
Copyright © 2022 Pei-Chin Lin et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
References
-
- Centers for Disease Control, Taiwan National Infectious Disease Statistics System . Centers for Disease Control, Atlanta, GA, USA; 2022, https://nidss.cdc.gov.tw/nndss/disease?id=19CoV.
LinkOut - more resources
Full Text Sources
Miscellaneous

