A Phase I, Single Ascending Dose Study of GEM103 (Recombinant Human Complement Factor H) in Patients with Geographic Atrophy
- PMID: 36249705
- PMCID: PMC9559901
- DOI: 10.1016/j.xops.2022.100154
A Phase I, Single Ascending Dose Study of GEM103 (Recombinant Human Complement Factor H) in Patients with Geographic Atrophy
Abstract
Purpose: To establish the safety, tolerability, pharmacokinetics, and pharmacodynamics of an intravitreal injection of recombinant human complement factor H (CFH), GEM103, in individuals with genetically defined age-related macular degeneration (AMD) and geographic atrophy (GA).
Design: Phase I single ascending-dose, open-label clinical trial (ClinicalTrials.gov identifier, NCT04246866).
Participants: Twelve individuals 50 years of age or older with a confirmed diagnosis of foveal GA in the study eye.
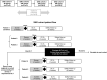
Methods: Participants were assigned to the increasing dose cohorts and received 1 50-μl intravitreal injection of GEM103 at doses of 50 μg/eye, 100 μg/eye, 250 μg/eye, or 500 μg/eye; dose escalation was dependent on the occurrence of dose-limiting toxicities.
Main outcome measures: Safety assessments included ocular and systemic adverse events (AEs), ocular examinations, clinical laboratory and vital signs, and serum antidrug antibody levels. Biomarkers, measured in the aqueous humor (AH), included CFH and complement activation biomarkers factor Ba and complement component 3a.
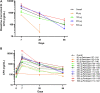
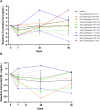
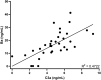
Results: No dose-limiting toxicities were reported, enabling escalation to the maximum study dose. No anti-GEM103 antidrug antibodies were detected during the study. Four participants experienced AEs; these were nonserious, mild or moderate in severity, and unrelated to GEM103. The AEs in 2 of these participants were related to the intravitreal injection procedure. No clinically significant ophthalmic changes and no ocular inflammation were observed. Visual acuity was maintained and stable throughout the 8-week follow-up period. No choroidal neovascularization occurred. CFH levels increased in a dose-dependent manner after GEM103 administration with supraphysiological levels observed at week 1; levels were more than baseline for 8 weeks or more in all participants receiving single doses of 100 μg or more. Complement activation biomarkers were reduced 7 days after dose administration.
Conclusions: A single intravitreal administration of GEM103 (up to 500 μg/eye) was well tolerated in individuals with GA. Of the few mild or moderate AEs reported, none were determined to be related to GEM103. No intraocular inflammation or choroidal neovascularization developed. CFH levels in AH were increased and stable for 8 weeks, with pharmacodynamic data suggesting that GEM103 restored complement regulation. These results support further development in a repeat-dose trial in patients with GA with AMD.
Keywords: AE, adverse event; AH, aqueous humor; AMD, age-related macular degeneration; Age-related macular degeneration; BCVA, best-corrected visual acuity; C3(a/b), complement component 3(a/b); CFB, complement factor B; CFH, complement factor H; CFP, color fundus photography; Complement factor H; Complement regulation; FA, fluorescein angiography; FAF, fundus autofluorescence; GA, geographic atrophy; GEM103; Geographic atrophy; IRC, image reading center; LLVA, low-luminance visual acuity; NI, near infrared; OCTA, optical coherence tomography angiography; RPE, retinal pigment epithelium; SD, standard deviation; nAMD, neovascular age-related macular degeneration.
© 2022 by the American Academy of Ophthalmology.
Figures

References
-
- Wong W.L., Su X., Li X., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–e116. - PubMed
-
- Bressler N.M. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901. - PubMed
-
- Schmitz-Valckenberg S., Sahel J.A., Danis R., et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study) Ophthalmology. 2016;123:361–368. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

