Development of mucoadhesive adapalene gel for biotherapeutic delivery to vaginal tissue
- PMID: 36249754
- PMCID: PMC9557122
- DOI: 10.3389/fphar.2022.1017549
Development of mucoadhesive adapalene gel for biotherapeutic delivery to vaginal tissue
Abstract
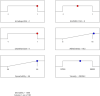
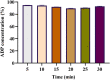
Purpose: Alternate formulation strategies need to be devised for improving the absorption and bioavailability of drug molecules administered through the intravaginal route. Enhancing the coating of vaginal mucosa can aid the achievement of this goal. The aim of the current study is to develop a mucoadhesive formulation having adequate adhesiveness, spreading, and viscosity profiles that can ensure good tissue absorption of adapalene upon intravaginal application. Method: A combination of mucoadhesive agents has been employed, including Carbopol-934, HPMC K-15M, and xanthan gum, in varying ratios to formulate five different gels. Furthermore, a cost-effective UV-spectroscopic analytical method was developed to quantify the amount of adapalene in tested samples, both of in vitro and in vivo origin. The analytical method was validated for different parameters, including specificity, linearity, range, accuracy, precision, and ruggedness. The modified USP-II apparatus was used for dissolution studies, while in vivo pharmacokinetic validation was performed in a murine model. Result: Of all the tested formulations, on the basis of the rheo-mechanical attributes, ACX3 performed better than the rest, including the commercially available intravaginal reference product. ACX3 had an average adhesion time of 12 min and a spread diameter of 37 mm. It showed 35 mm as average distance travelled by the diluted sample for leakage assessment. The analytical method developed for the adapalene muco-adhesive gel was within the range for all the validation parameters. For further evaluating the performance of the formulation, dissolution studies were conducted in simulated vaginal conditions which showed 94.83% of drug release within 5 minutes, while on completion of 30 min, it was measured to be 92.90%. Moreover, approximately 67% of the administered drug was recovered after 5 min of administration as evaluated through tissue recovery procedures in mice. Conclusion: The study aided in development of a formulation which can enhance the muco-adhesion of the drug molecule, resulting in an improved pharmacokinetic profile. Moreover, it established an efficient assay method which can be employed for in vitro and in vivo quantification of adapalene in simulated and physiological fluids.
Keywords: adapalene; assay development; dissolution; intravaginal drug delivery; mucoadhesion.
Copyright © 2022 Afzaal, Shahiq-uz-Zaman, Saeed, Hamdani, Raza, Gul, Babar and Rajadas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alawdi S., Solanki A. B. (2021). Mucoadhesive drug delivery systems: A review of recent developments. J. Sci. Res. Med. Biol. Sci. 2, 50–64. 10.47631/jsrmbs.v2i1.213 - DOI
-
- Andrade A. O., Parente M. E., Ares G. (2014). Screening of mucoadhesive vaginal gel formulations. Braz. J. Pharm. Sci. 50, 931–941. 10.1590/s1984-82502014000400029 - DOI
-
- Atole D. M., Rajput H. H. (2018). Ultraviolet spectroscopy and its pharmaceutical applications-a brief review. Asian J. Pharm. Clin. Res. 11, 59–66. 10.22159/ajpcr.2018.v11i2.21361 - DOI
LinkOut - more resources
Full Text Sources

