Atovaquone for treatment of COVID-19: A prospective randomized, double-blind, placebo-controlled clinical trial
- PMID: 36249792
- PMCID: PMC9561237
- DOI: 10.3389/fphar.2022.1020123
Atovaquone for treatment of COVID-19: A prospective randomized, double-blind, placebo-controlled clinical trial
Abstract
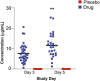
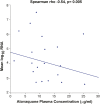
Background: An in silico screen was performed to identify FDA approved drugs that inhibit SARS-CoV-2 main protease (Mpro), followed by in vitro viral replication assays, and in vivo pharmacokinetic studies in mice. These studies identified atovaquone as a promising candidate for inhibiting viral replication. Methods: A 2-center, randomized, double-blind, placebo-controlled trial was performed among patients hospitalized with COVID-19 infection. Enrolled patients were randomized 2:1 to atovaquone 1500 mg BID versus matched placebo. Patients received standard of care treatment including remdesivir, dexamethasone, or convalescent plasma as deemed necessary by the treating team. Saliva was collected at baseline and twice per day for up to 10 days for RNA extraction for SARS-CoV-2 viral load measurement by quantitative reverse-transcriptase PCR. The primary outcome was the between group difference in log-transformed viral load (copies/mL) using a generalized linear mixed-effect models of repeated measures from all samples. Results: Of the 61 patients enrolled; 41 received atovaquone and 19 received placebo. Overall, the population was predominately male (63%) and Hispanic (70%), with a mean age of 51 years, enrolled a mean of 5 days from symptom onset. The log10 viral load was 5.25 copies/mL vs. 4.79 copies/mL at baseline in the atovaquone vs. placebo group. Change in viral load did not differ over time between the atovaquone plus standard of care arm versus the placebo plus standard of care arm. Pharmacokinetic (PK) studies of atovaquone plasma concentration demonstrated a wide variation in atovaquone levels, with an inverse correlation between BMI and atovaquone levels, (Rho -0.45, p = 0.02). In post hoc analysis, an inverse correlation was observed between atovaquone levels and viral load (Rho -0.54, p = 0.005). Conclusion: In this prospective, randomized, placebo-controlled trial, atovaquone did not demonstrate evidence of enhanced SARS-CoV-2 viral clearance compared with placebo. However, based on the observed inverse correlation between atovaquone levels and viral load, additional PK-guided studies may be warranted to examine the antiviral effect of atovaquone in COVID-19 patients.
Keywords: COVID-19; atovaquone; clinical trial; double blind; placebo controlled.
Copyright © 2022 Jain, De Lemos, McGuire, Ayers, Eitson, Sanchez, Kamel, Meisner, Thomas, Hegde, Mocherla, Strebe, Li, Williams, Xing, Ahmed, Wang, Sadek and Schoggins.
Conflict of interest statement
MKJ has received research funding from Gilead Sciences and Regeneron and was on Advisory Board for Gilead Sciences. SM received research funding from Regeneron. JADL has received consulting income from Regeneron and Eli Lilly unrelated to COVID-19. JWS serves as a consultant for the Federal Trade Commission on matters related to COVID-19 treatments. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahmed M., Wang P., Boys I. N., Eitson J. L., Ohlson M. B., Fan W., et al. (2021). Identification of atovaquone as and mebendazole as repurposed drugs with antiviral activity against SARS-CoV-2 chemRxiv. Available at: https://chemrxiv.org/engage/chemrxiv/article-details/612ff2f8abeb6328b6c... .
-
- Davey R. T., Jr., Fernandez-Cruz E., Markowitz N., Pett S., Babiker A. G., Wentworth D., et al. (2019). Anti-influenza hyperimmune intravenous immunoglobulin for adults with influenza A or B infection (FLU-IVIG): A double-blind, randomised, placebo-controlled trial. Lancet. Respir. Med. 7, 951–963. 10.1016/S2213-2600(19)30253-X - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

