Physiologically-based pharmacokinetic modelling and dosing evaluation of gentamicin in neonates using PhysPK
- PMID: 36249803
- PMCID: PMC9554458
- DOI: 10.3389/fphar.2022.977372
Physiologically-based pharmacokinetic modelling and dosing evaluation of gentamicin in neonates using PhysPK
Abstract
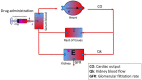
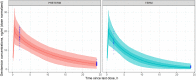
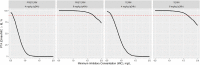
Each year, infections caused around the 25% of neonatal deaths. Early empirical treatments help to reduce this mortality, although optimized dosing regimens are still lacking. The aims were to develop and validate a gentamicin physiologically-based pharmacokinetic (PBPK) model and then potentially explore dosing regimens in neonates using pharmacokinetic and pharmacodynamic criteria. The PBPK model developed consisted of 2 flow-limited tissues: kidney and other tissues. It has been implemented on a new tool called PhysPK, which allows structure reusability and evolution as predictive engine in Model-Informed Precision Dosing (MIPD). Retrospective pharmacokinetic information based on serum levels data from 47 neonates with gestational age between 32 and 39 weeks and younger than one-week postnatal age were used for model validation. The minimal PBPK model developed adequately described the gentamicin serum concentration-time profile with an average fold error nearly 1. Extended interval gentamicin dosing regimens (6 mg/kg q36h and 6 mg/kg q48h for term and preterm neonates, respectively) showed efficacy higher than 99% with toxicity lower than 10% through Monte Carlo simulation evaluations. The gentamicin minimal PBPK model developed in PhysPK from literature information, and validated in preterm and term neonates, presents adequate predictive performance and could be useful for MIPD strategies in neonates.
Keywords: PBPK; PhysPK software; TDM; dosing evaluation; gentamicin; neonates.
Copyright © 2022 Zazo, Lagarejos, Prado-Velasco, Sánchez-Herrero, Serna, Rueda-Ferreiro, Martín-Suárez, Calvo, Pérez-Blanco and Lanao.
Conflict of interest statement
Preliminary results of this research were presented at XIV Jornadas de modelización y simulación en biomedicina, in November 2021 in Barcelona, Spain. SS-H, JS and AR-F were employees of “Empresarios Agrupados SA” at the time the analysis was conducted. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- ANMF - Australasian Neonatal Medicines Formulary (2022). Anmf - australasian neonatal Medicines formulary. [Internet][citado 17 de marzo de 2022]. Disponible en: Available at: https://www.anmfonline.org/ (Accessed March 17, 2022).
-
- Comité de Medicamentos de la Asociación Española de Pediatríaá, 2022, Pediamécum. Edición 2015 | Asociación Española de Pediatría; [Internet]. [citado 7 de febrero de 2022]. Disponible en: Available at: https://www.aeped.es/comite-medicamentos/pediamecum/gentamicina (Accessed February 7, 2022).
LinkOut - more resources
Full Text Sources

