Site-Selective Modification of (Oligo)Saccharides
- PMID: 36249871
- PMCID: PMC9552177
- DOI: 10.1021/acscatal.2c03876
Site-Selective Modification of (Oligo)Saccharides
Abstract
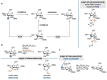
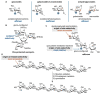
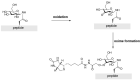
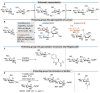
Oligosaccharides, either as such or as part of glycolipids, glycopeptides, or glycoproteins, are ubiquitous in nature and fulfill important roles in the living cell. Also in medicine and to some extent in materials, oligosaccharides play an important role. In order to study their function, modifying naturally occurring oligosaccharides, and building in reactive groups and reporter groups in oligosaccharides, are key strategies. The development of oligosaccharides as drugs, or vaccines, requires the introduction of subtle modifications in the structure of oligosaccharides to optimize efficacy and, in the case of antibiotics, circumvent bacterial resistance. Provided the natural oligosaccharide is available, site-selective modification is an attractive approach as total synthesis of the target is often very laborious. Researchers in catalysis areas, such as transition-metal catalysis, enzyme catalysis, organocatalysis, and photoredox catalysis, have made considerable progress in the development of site-selective and late-stage modification methods for mono- and oligosaccharides. It is foreseen that the fields of enzymatic modification of glycans and the chemical modification of (oligo)saccharides will approach and potentially meet each other, but there is a lot to learn and discover before this will be the case.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Börgel J.; Ritter T. Late-Stage Functionalisation. Chem. 2020, 6, 1877–1887. 10.1016/j.chempr.2020.07.007. - DOI
-
- For an interesting review on the selective partial protection of monosaccharides, see:Wang T.; Demchenko A. V. Synthesis of carbohydrate building blocks via regioselective uniform protection/deprotection strategies. Org. Biomol. Chem. 2019, 17, 4934–4950. 10.1039/C9OB00573K. - DOI - PMC - PubMed
- For an overview of the use of non-valent interactions, see:Loh C. C. J. Exploiting non-covalent interactions in selective carbohydrate synthesis. Nat. Rev. Chem. 2021, 5, 792–815. 10.1038/s41570-021-00324-y. - DOI - PubMed
-
- Tsuda Y.; Hanajima M.; Matsuhira N.; Okuno Y.; Kanemitsu K. Regioselective Mono-oxidation of Non-protected Carbohydrates by Brominolysis of the Tin Intermediates. Chem. Pharm. Bull. 1989, 37, 2344–2350. 10.1248/cpb.37.2344. - DOI
Publication types
LinkOut - more resources
Full Text Sources
