Optimization of the Cetyltrimethylammonium bromide (CTAB) DNA extraction protocol using forest elephant dung samples
- PMID: 36249934
- PMCID: PMC9558105
- DOI: 10.1016/j.mex.2022.101867
Optimization of the Cetyltrimethylammonium bromide (CTAB) DNA extraction protocol using forest elephant dung samples
Abstract
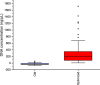
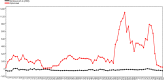
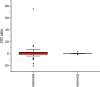
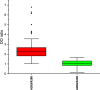
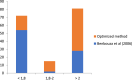
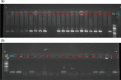
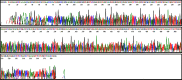
Among non-invasive biological samples, feces offer an important source of DNA and can easily be collected. However, working with fecal DNA from highly vegetarians species such as elephant is more challenging because plant secondary compounds have an inhibitory effect on PCR reactions. Working with forest elephant dung samples, we tested and adapted a protocol of DNA extraction developed on plants based on the Cetyltrimethylammonium bromide (CTAB) protocol. The protocol is relatively simple and yields a high DNA concentration. It is five-time less expensive compared to the methods of Benbouza et al. The extracted DNA is of good quality and easily amplified by PCR. The high-amplification percentage of mitochondrial genes in fecal DNA and subsequent sequencing of PCR products indicate that the proposed optimized method is reliable for molecular analysis of forest elephant dung samples.•Our optimized CTAB protocol has been adjusted by the addition of Sodium Dodecyl Sulfate (SDS) and proteinase K during the lysis phase. The combined effect of these reagents was capable of lysing cell walls and removing proteins efficiently.•Moreover, the prolonged time of incubation (overnight incubation at room temperature followed by 3 hours of incubation in a water bath) enhanced the increase of DNA yield but make the optimized protocol more time-consuming.
Keywords: DNA quality and quantity; Fecal sample; Improved CTAB method; Non-invasive sample; PCR success.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Taberlet P., Waits L.P., Luikart G. Noninvasive genetic sampling: look before you leap. Trends Ecol. Evol. 1999;14:323‑27. - PubMed
-
- Hoss M., Kohn M., Abo P., Knauer S.F., Schroder W. Excrement analysis by PCR. Nature. 1992;359:199. - PubMed
-
- Eggert L.S., Eggert J.A., Woodruff D.S. Estimating population sizes for elusive animals: the forest elephants of Kakum National Park, Ghana. Mol. Ecol. 2003;12:1389‑1402. - PubMed
-
- Lantz M.E., Ronald A.C., Rodriguez A., Kathy B.P. Maternal weight gain patterns and birth weight outcome in twin gestation. Obstetr. Gynecol. 1996;87:551‑56. - PubMed
LinkOut - more resources
Full Text Sources

