Anti-cancer potency by induced apoptosis by molecular docking P53, caspase, cyclin D1, cytotoxicity analysis and phagocytosis activity of trisindoline 1,3 and 4
- PMID: 36249936
- PMCID: PMC9563049
- DOI: 10.1016/j.jsps.2022.06.012
Anti-cancer potency by induced apoptosis by molecular docking P53, caspase, cyclin D1, cytotoxicity analysis and phagocytosis activity of trisindoline 1,3 and 4
Abstract
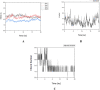
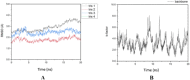
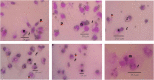
Cancer is one of the leading causes of death in the world. Efforts to find and develop cancer drugs from natural products continue with the exploration of trisindoline, a substance that is isolated from marine sponges Hyrtios altum. Trisindoline is an indole trimer alkaloid compound that has been successfully synthesized into trisindoline 1, 3 and 4. Trisindoline is cytotoxic in cell lines and in this study, trisindoline was able to induce apoptosis in the in silico and in vitro tests that were carried out. The in silico test was carried out through molecular docking using the Autodock Vina method and the Molecular Dynamics (MD) Simulation QM / MM AMBER. The target proteins used were protein p53 and caspase -9 which played a role in the apoptotic pathway and cyclin D1 which played a role in cell proliferation. Meanwhile, cytotoxicity analysis was carried out using the MTT method (3- (4,5-dimethyltiazol -2-yl) -2,5 -dipenyl tetrazolium bromide). Nevertheless, the ability of trisindoline to induce phagocytosis is still unrevealed. The phagocytosis assay was carried out by assessing the macrophage capacity and phagocytic index using the latex-beads model. The in silico results showed that the binding affinity values between the target protein Cdk-2 and the trisindoline 1, trisindoline 3 and trisindoline 4 ligands were -7.3 kcal / mol, -7.7 kcal / mol and -6.6 kcal / mol respectively. The binding affinity values between the target protein p53 and the trisindoline 1, trisindoline 3 and trisindoline 4 ligands were -7.5 kcal / mol, -7.4 kcal / mol and -7.5 kcal / mol respectively. The binding affinity values between the target protein caspase-9 and the trisindoline 1, trisindoline 3 and trisindoline 4 ligands were -7.5 kcal / mol, -7.1 kcal / mol and -7.2 kcal / mol respectively. The results of RMSD (Root Mean Square Deviation), RMSF (Root Mean Square Fluctuation), and hydrogen bonds in the MD (Molecular Dynamics) Simulation showed that Cdk-2 formed a protein complex with trisindoline 3, protein p53 with trisindoline 1 and caspase-9 with trisindoline 1. The cytotoxicity assay was carried out in the MCF-7 cell line and the IC50 value obtained for trisindoline 1 was 2.059 μM, for trisindoline 3 was 3.9759 μM, for trisindoline 4 was 15.46 μM and for doxorubicin was 9.88 μM. Furthermore, the phagocytosis test was carried out using trisindoline 1, 3 and 4. Our results showed that 6.25 µg mL-1 of trisindoline 1 and trisindoline 3 were able to induce the phagocytosis capacity of macrophage cells of 38.34; whereas trisindoline 4 at a concentration of 50 µg mL-1 induces a phagocytosis capacity of 32.89. Trisindoline 1, 3 and 4 showed potentials of immunostimulants at low concentrations but showed potentials of immunosuppressants at high concentrations. The overall results demonstrated that trisindoline 1 and 3 are potential anti-cancer candidates capable of activating the apoptotic pathway.
Keywords: Caspase 9; In Silico; MCF-7; P53; Trisindoline.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









Similar articles
-
Synthesis, Molecular Docking, Molecular Dynamics Studies, and Biological Evaluation of 4H-Chromone-1,2,3,4-tetrahydropyrimidine-5-carboxylate Derivatives as Potential Antileukemic Agents.J Chem Inf Model. 2017 Jun 26;57(6):1246-1257. doi: 10.1021/acs.jcim.6b00138. Epub 2017 May 25. J Chem Inf Model. 2017. PMID: 28524659
-
Exploration of binding mechanism of triclosan towards cancer markers using molecular docking and molecular dynamics.Chemosphere. 2022 Apr;293:133550. doi: 10.1016/j.chemosphere.2022.133550. Epub 2022 Jan 6. Chemosphere. 2022. PMID: 34999105
-
Investigation of the New Inhibitors by Sulfadiazine and Modified Derivatives of α-D-glucopyranoside for White Spot Syndrome Virus Disease of Shrimp by In Silico: Quantum Calculations, Molecular Docking, ADMET and Molecular Dynamics Study.Molecules. 2022 Jun 8;27(12):3694. doi: 10.3390/molecules27123694. Molecules. 2022. PMID: 35744817 Free PMC article.
-
Target-based virtual screening, computational multiscoring docking and molecular dynamics simulation of small molecules as promising drug candidate affecting kinesin-like protein KIFC1.Cell Biochem Funct. 2022 Jul;40(5):451-472. doi: 10.1002/cbf.3707. Epub 2022 Jun 27. Cell Biochem Funct. 2022. PMID: 35758564
-
Marine natural products. XXXIV. Trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum.Chem Pharm Bull (Tokyo). 1994 Dec;42(12):2449-51. doi: 10.1248/cpb.42.2449. Chem Pharm Bull (Tokyo). 1994. PMID: 7697760
Cited by
-
A comprehensive apoptotic assessment of niloticin in cervical cancer cells: a tirucallane-type triterpenoid from Aphanamixis polystachya (Wall.) Parker.RSC Med Chem. 2024 Aug 8;15(10):3444-59. doi: 10.1039/d4md00318g. Online ahead of print. RSC Med Chem. 2024. PMID: 39246746 Free PMC article.
-
Exploration of the anticancer efficacy of a novel 1,3-thiazole analog in an ehrlich ascites carcinoma model: in vivo and in silico insights into hepatorenal protective potentials via the modulation of apoptosis, oxidative stress and inflammation.RSC Adv. 2025 Jun 13;15(25):20143-20167. doi: 10.1039/d5ra01014d. eCollection 2025 Jun 10. RSC Adv. 2025. PMID: 40519680 Free PMC article.
-
Analysing the Anticancer Properties of Pterostilbene Through Absorption, Distribution, Metabolism, and Excretion (ADME) and Molecular Docking Studies.Cureus. 2024 Apr 16;16(4):e58425. doi: 10.7759/cureus.58425. eCollection 2024 Apr. Cureus. 2024. PMID: 38756274 Free PMC article.
References
-
- Arwansyah, Ambarsari L., Sumaryada T.I. Simulasi Docking Senyawa Kurkumin dan Analognya Sebagai Inhibitor Reseptor Androgen pada Kanker Prostat. Curr. Biochem. 2014;1:11–19. doi: 10.29244/cb.1.1.11-19. - DOI
-
- Bai L., Zhu W.-G. p53: Structure, Function dan Therapeutic Applications. J. Cancer Mol. 2006;2:141–153.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

