Two novel bombesin-like neuropeptides from the skin secretion of Pelophylax kl. esculentus: Ex vivo pharmacological characterization on rat smooth muscle types
- PMID: 36250016
- PMCID: PMC9560764
- DOI: 10.3389/fmolb.2022.953974
Two novel bombesin-like neuropeptides from the skin secretion of Pelophylax kl. esculentus: Ex vivo pharmacological characterization on rat smooth muscle types
Abstract
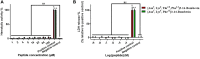
Mammalian bombesin-like neuropeptides (BLPs) play an important role in regulation of physiological and pathophysiological processes. Frog skin-derived BLPs, of smaller size and diverse lengths and sequences at their N-terminus, have attracted the attention of many researchers. However, these N-terminal variants and the receptors modulating their pharmacological actions are poorly studied and less understood. In this study, two BLPs, namely, [Asn3, Lys6, Thr10, Phe13]3-14-bombesin and [Asn3, Lys6, Phe13]3-14-bombesin with primary structures NLGKQWATGHFM and NLGKQWAVGHFM were isolated from the skin secretion of hybrid Pelophylax kl. esculentus. Both BLPs share a similar primary structure with only a single amino acid substitution at the eighth position (threonine to valine), while they have quite different myotropic potencies with EC50 values in the range of 22.64 ± 9.7 nM (N = 8) to 83.93 ± 46.9 nM (N = 8). The potency of [Asn3, Lys6, Thr10, Phe13]3-14-bombesin was approximately 3-fold higher than that of [Asn3, Lys6, Phe13]3-14-bombesin. Through the investigation of receptor selectivity using a canonical bombesin receptor antagonist, it was found that [Asn3, Lys6, Thr10, Phe13]3-14-bombesin and [Asn3, Lys6, Phe13]3-14-bombesin had an affinity to both BB1 and BB2 receptors. Their contractile functions are mainly modulated by both BB1 and BB2 receptors on rat urinary bladder and BB2 alone on rat uterus smooth muscle preparations. These data may provide new insights into the design of potent and selective ligands for bombesin receptors. Moreover, [Asn3, Lys6, Thr10, Phe13]3-14-bombesin and [Asn3, Lys6, Phe13]3-14-bombesin did not induce significant hemolysis and toxicity in normal human cells, suggesting that these two natural novel BLPs have great potential for development into new drug candidates.
Keywords: N-terminal variants; bombesin receptor; bombesin-like neuropeptides (BLPs); frog skin; molecule cloning; pharmacological properties; smooth muscle.
Copyright © 2022 Zhang, Chen, Zou, Chen, Zhou, Ma, Xi, Chen, Shaw, Liu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Accardo A., Mannucci S., Nicolato E., Vurro F., Diaferia C., Bontempi P., et al. (2019). Easy formulation of liposomal doxorubicin modified with a bombesin peptide analogue for selective targeting of GRP receptors overexpressed by cancer cells. Drug Deliv. Transl. Res. 9, 215–226. 10.1007/s13346-018-00606-x - DOI - PubMed
-
- Bai B., Wang H., Xue Y., Wu Y., Zhou M., Wei M., et al. (2012). The structures of four bombesins and their cloned precursor-encoding cDNAs from acid-solvated skin secretion of the European yellow-bellied toad, Bombina variegata . Peptides 36 (2), 221–229. 10.1016/j.peptides.2012.05.020 - DOI - PubMed
-
- Bardin C. W. (1993). Recent progress in hormone research: Proceedings of the 1991 laurentian hormone conference, 48. Saint Louis: Elsevier Scienvier & Technology, 1–553.
LinkOut - more resources
Full Text Sources
Miscellaneous

