Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases
- PMID: 36250046
- PMCID: PMC9559808
- DOI: 10.3389/fcimb.2022.987683
Implications of Porphyromonas gingivalis peptidyl arginine deiminase and gingipain R in human health and diseases
Abstract
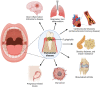
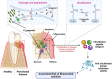
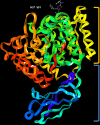
Porphyromonas gingivalis is a major pathogenic bacterium involved in the pathogenesis of periodontitis. Citrullination has been reported as the underlying mechanism of the pathogenesis, which relies on the interplay between two virulence factors of the bacterium, namely gingipain R and the bacterial peptidyl arginine deiminase. Gingipain R cleaves host proteins to expose the C-terminal arginines for peptidyl arginine deiminase to citrullinate and generate citrullinated proteins. Apart from carrying out citrullination in the periodontium, the bacterium is found capable of citrullinating proteins present in the host synovial tissues, atherosclerotic plaques and neurons. Studies have suggested that both virulence factors are the key factors that trigger distal effects mediated by citrullination, leading to the development of some non-communicable diseases, such as rheumatoid arthritis, atherosclerosis, and Alzheimer's disease. Thus, inhibition of these virulence factors not only can mitigate periodontitis, but also can provide new therapeutic solutions for systematic diseases involving bacterial citrullination. Herein, we described both these proteins in terms of their unique structural conformations and biological relevance to different human diseases. Moreover, investigations of inhibitory actions on the enzymes are also enumerated. New approaches for identifying inhibitors for peptidyl arginine deiminase through drug repurposing and virtual screening are also discussed.
Keywords: Porphyromonas gingivalis; citrullination; gingipain R; inhibitors; peptidyl arginine deiminase; systemic disease.
Copyright © 2022 Chow, Yam, Gunasekaran, Lai, Wo, Agarwal, Ong, Cheong and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Abe N., Kadowaki T., Okamoto K., Nakayama K., Ohishi M., Yamamoto K. (1998). Biochemical and functional properties of lysine-specific cysteine proteinase (Lys-gingipain) as a virulence factor of porphyromonas gingivalis in periodontal disease. J. Biochem. 123 (2), 305–312. doi: 10.1093/oxfordjournals.jbchem.a021937 - DOI - PubMed
-
- Alhogail S., Suaifan G., Bizzarro S., Kaman W. E., Bikker F. J., Weber K., et al. . (2018). On site visual detection of porphyromonas gingivalis related periodontitis by using a magnetic-nanobead based assay for gingipains protease biomarkers. Microchim. Acta 185 (2), 149. doi: 10.1007/s00604-018-2677-x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

