Complementary crosstalk between palmitoylation and phosphorylation events in MTIP regulates its role during Plasmodium falciparum invasion
- PMID: 36250062
- PMCID: PMC9556994
- DOI: 10.3389/fcimb.2022.924424
Complementary crosstalk between palmitoylation and phosphorylation events in MTIP regulates its role during Plasmodium falciparum invasion
Abstract
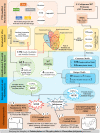
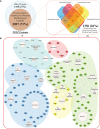
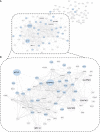
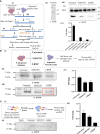
Post-translational modifications (PTMs) including phosphorylation and palmitoylation have emerged as crucial biomolecular events that govern many cellular processes including functioning of motility- and invasion-associated proteins during Plasmodium falciparum invasion. However, no study has ever focused on understanding the possibility of a crosstalk between these two molecular events and its direct impact on preinvasion- and invasion-associated protein-protein interaction (PPI) network-based molecular machinery. Here, we used an integrated in silico analysis to enrich two different catalogues of proteins: (i) the first group defines the cumulative pool of phosphorylated and palmitoylated proteins, and (ii) the second group represents a common set of proteins predicted to have both phosphorylation and palmitoylation. Subsequent PPI analysis identified an important protein cluster comprising myosin A tail interacting protein (MTIP) as one of the hub proteins of the glideosome motor complex in P. falciparum, predicted to have dual modification with the possibility of a crosstalk between the same. Our findings suggested that blocking palmitoylation led to reduced phosphorylation and blocking phosphorylation led to abrogated palmitoylation of MTIP. As a result of the crosstalk between these biomolecular events, MTIP's interaction with myosin A was found to be abrogated. Next, the crosstalk between phosphorylation and palmitoylation was confirmed at a global proteome level by click chemistry and the phenotypic effect of this crosstalk was observed via synergistic inhibition in P. falciparum invasion using checkerboard assay and isobologram method. Overall, our findings revealed, for the first time, an interdependence between two PTM types, their possible crosstalk, and its direct impact on MTIP-mediated invasion via glideosome assembly protein myosin A in P. falciparum. These insights can be exploited for futuristic drug discovery platforms targeting parasite molecular machinery for developing novel antimalarial therapeutics.
Keywords: crosstalk; malaria; myosin A tail interacting protein (MTIP); plasmodium falciparum; post-translational modifications.
Copyright © 2022 Anam, Kumari, Mukherjee, Rex, Biswas, Maurya, Ravikumar, Gupta, Kushawaha, Sah, Chaurasiya, Singhal, Singh, Kaushik, Prasad, Pati, Ranganathan and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A De novo Peptide from a High Throughput Peptide Library Blocks Myosin A -MTIP Complex Formation in Plasmodium falciparum.Int J Mol Sci. 2020 Aug 26;21(17):6158. doi: 10.3390/ijms21176158. Int J Mol Sci. 2020. PMID: 32859024 Free PMC article.
-
The MTIP-myosin A complex in blood stage malaria parasites.J Mol Biol. 2006 Feb 3;355(5):933-41. doi: 10.1016/j.jmb.2005.11.027. Epub 2005 Nov 28. J Mol Biol. 2006. PMID: 16337961
-
Regulation of the Plasmodium motor complex: phosphorylation of myosin A tail-interacting protein (MTIP) loosens its grip on MyoA.J Biol Chem. 2012 Oct 26;287(44):36968-77. doi: 10.1074/jbc.M112.379842. Epub 2012 Aug 29. J Biol Chem. 2012. PMID: 22932904 Free PMC article.
-
Palmitoylation and palmitoyl-transferases in Plasmodium parasites.Biochem Soc Trans. 2015 Apr;43(2):240-5. doi: 10.1042/BST20140289. Biochem Soc Trans. 2015. PMID: 25849924 Review.
-
Emerging roles for protein S-palmitoylation in Toxoplasma biology.Int J Parasitol. 2014 Feb;44(2):121-31. doi: 10.1016/j.ijpara.2013.09.004. Epub 2013 Nov 1. Int J Parasitol. 2014. PMID: 24184909 Review.
References
-
- Akoachere M., Buchholz K., Fischer E., Burhenne J., Haefeli W. E., Schirmer R. H., et al. . (2005). In vitro assessment of methylene blue on chloroquine-sensitive and -resistant plasmodium falciparum strains reveals synergistic action with artemisinins. Antimicrob. Agents Chemother. 49, 4592. doi: 10.1128/AAC.49.11.4592-4597.2005 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

