An update on preclinical models of hereditary haemorrhagic telangiectasia: Insights into disease mechanisms
- PMID: 36250069
- PMCID: PMC9556665
- DOI: 10.3389/fmed.2022.973964
An update on preclinical models of hereditary haemorrhagic telangiectasia: Insights into disease mechanisms
Abstract
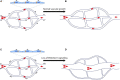
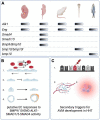
Endoglin (ENG) is expressed on the surface of endothelial cells (ECs) where it efficiently binds circulating BMP9 and BMP10 ligands to initiate activin A receptor like type 1 (ALK1) protein signalling to protect the vascular architecture. Patients heterozygous for ENG or ALK1 mutations develop the vascular disorder known as hereditary haemorrhagic telangiectasia (HHT). Many patients with this disorder suffer from anaemia, and are also at increased risk of stroke and high output heart failure. Recent work using animal models of HHT has revealed new insights into cellular and molecular mechanisms causing this disease. Loss of the ENG (HHT1) or ALK1 (HHT2) gene in ECs leads to aberrant arteriovenous connections or malformations (AVMs) in developing blood vessels. Similar phenotypes develop following combined EC specific loss of SMAD1 and 5, or EC loss of SMAD4. Taken together these data point to the essential role of the BMP9/10-ENG-ALK1-SMAD1/5-SMAD4 pathway in protecting the vasculature from AVMs. Altered directional migration of ECs in response to shear stress and increased EC proliferation are now recognised as critical factors driving AVM formation. Disruption of the ENG/ALK1 signalling pathway also affects EC responses to vascular endothelial growth factor (VEGF) and crosstalk between ECs and vascular smooth muscle cells. It is striking that the vascular lesions in HHT are both localised and tissue specific. Increasing evidence points to the importance of a second genetic hit to generate biallelic mutations, and the sporadic nature of such somatic mutations would explain the localised formation of vascular lesions. In addition, different pro-angiogenic drivers of AVM formation are likely to be at play during the patient's life course. For example, inflammation is a key driver of vessel remodelling in postnatal life, and may turn out to be an important driver of HHT disease. The current wealth of preclinical models of HHT has led to increased understanding of AVM development and revealed new therapeutic approaches to treat AVMs, and form the topic of this review.
Keywords: BMP-SMAD signalling pathway; HHT; activin receptor-like kinase 1 (ACVRL1); arteriovenous malformation (AVM); cell polarity and migration; endoglin (CD105).
Copyright © 2022 Arthur and Roman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, et al. Endoglin, a Tgf-Beta Binding Protein of Endothelial Cells, Is the Gene for Hereditary Haemorrhagic Telangiectasia Type 1. Nat Genet. (1994) 8:345–51. - PubMed
-
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, Yoon SJ, et al. Mutations in the Activin Receptor-Like Kinase 1 Gene in Hereditary Haemorrhagic Telangiectasia Type 2. Nat Genet. (1996) 13:189–95. - PubMed
-
- Balachandar S, Graves TJ, Shimonty A, Kerr K, Kilner J, Xiao S, et al. Identification and Validation of a Novel Pathogenic Variant in Gdf2 (Bmp9) Responsible for Hereditary Hemorrhagic Telangiectasia and Pulmonary Arteriovenous Malformations. Am J Med Genet A. (2022) 188:959–64. 10.1002/ajmg.a.62584 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

