Survival outcomes of surgical and non-surgical treatment in elderly patients with stage I pancreatic cancer: A population-based analysis
- PMID: 36250070
- PMCID: PMC9556697
- DOI: 10.3389/fmed.2022.958257
Survival outcomes of surgical and non-surgical treatment in elderly patients with stage I pancreatic cancer: A population-based analysis
Abstract
Background: Due to underrepresentation in randomized controlled trials among old people (≥65 years old), the effectiveness of clinical trial-based recommendations about the treatment for stage I pancreatic cancer remains controversial. In this research, we intended to investigate the different strategies of this population in surgery group and non-surgery group.
Materials and methods: Elderly patients aged 65 years or older with histologically diagnosed stage I pancreatic cancer from 2006 to 2017 were identified from the Surveillance, Epidemiology, and End Results (SEER) database. The included patients were divided into surgery group (receiving surgery with chemotherapy or chemoradiotherapy) and non-surgery group (receiving radiotherapy, chemotherapy, both, or neither). Overall survival (OS) and cancer-specific survival (CSS) were compared between groups by Kaplan-Meier analysis. Cox proportional hazards regression (Cox) proportional hazards regression was used to determine factors associated with survival.
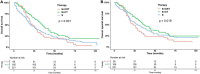
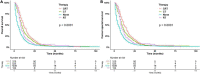
Results: A total of 2,448 eligible patients were recruited. Among them, 18.4% were treated surgically and 81.6% were treated non-surgically. The median OS (mOS) was 26 months (95% CI: 24-30 months) in the surgery group and 7 months (95% CI: 7-8 months) in the non-surgery group. In multivariate analyses, surgery was an important factor in improving OS compared with non-surgical treatment (HR: 0.34, 95% CI: 0.29-0.39, p < 0.001). In subgroup analysis, surgery plus chemotherapy was an independent factor for OS in the surgery group, while chemoradiotherapy, chemotherapy, and radiotherapy were independent prognostic factors for patients in the non-surgery group.
Conclusion: Surgical resection and post-operative chemotherapy are recommended for elderly patients with stage I pancreatic cancer who can tolerate treatment, but post-operative chemoradiotherapy does not bring survival benefits compared with post-operative chemotherapy. Moreover, radiotherapy, chemotherapy, or the combination of radiotherapy and chemotherapy are significantly related to the prognosis of elderly patients with untreated pancreatic cancer, but chemoradiotherapy has the most obvious benefit.
Keywords: SEER; chemotherapy; elderly; pancreatic cancer; radiation; surgery.
Copyright © 2022 Nie, Lan, Shi and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Can Elderly Patients With Pancreatic Cancer Gain Survival Advantages Through More Radical Surgeries? A SEER-Based Analysis.Front Oncol. 2020 Oct 29;10:598048. doi: 10.3389/fonc.2020.598048. eCollection 2020. Front Oncol. 2020. PMID: 33194764 Free PMC article.
-
The choice of adjuvant radiotherapy in pancreatic cancer patients after up-front radical surgery.PLoS One. 2025 Jan 24;20(1):e0317995. doi: 10.1371/journal.pone.0317995. eCollection 2025. PLoS One. 2025. PMID: 39854493 Free PMC article.
-
Effect of neoadjuvant radiotherapy on survival of non-metastatic pancreatic ductal adenocarcinoma: a SEER database analysis.Radiat Oncol. 2020 May 13;15(1):107. doi: 10.1186/s13014-020-01561-z. Radiat Oncol. 2020. PMID: 32404114 Free PMC article.
-
Survival benefits and disparities in radiation therapy for elderly patients with pancreatic ductal adenocarcinoma.World J Gastrointest Oncol. 2023 Jan 15;15(1):155-170. doi: 10.4251/wjgo.v15.i1.155. World J Gastrointest Oncol. 2023. PMID: 36684051 Free PMC article.
-
The Effectiveness of Chemoradiotherapy in Elderly Patients with Pancreatic Cancer: A Population-Based Study Based on the SEER Database.Adv Ther. 2022 Nov;39(11):5043-5057. doi: 10.1007/s12325-022-02297-w. Epub 2022 Aug 31. Adv Ther. 2022. PMID: 36044179
Cited by
-
Deciphering the role of NcRNAs in Pancreatic Cancer immune evasion and drug resistance: a new perspective for targeted therapy.Front Immunol. 2024 Nov 1;15:1480572. doi: 10.3389/fimmu.2024.1480572. eCollection 2024. Front Immunol. 2024. PMID: 39555076 Free PMC article. Review.
References
-
- Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet. (2017) 389:1011–24. 10.1016/S0140-6736(16)32409-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources

