Long-term Outcomes With Islet-Alone and Islet-After-Kidney Transplantation for Type 1 Diabetes in the Clinical Islet Transplantation Consortium: The CIT-08 Study
- PMID: 36250905
- PMCID: PMC9767903
- DOI: 10.2337/dc21-2688
Long-term Outcomes With Islet-Alone and Islet-After-Kidney Transplantation for Type 1 Diabetes in the Clinical Islet Transplantation Consortium: The CIT-08 Study
Abstract
Objective: To determine long-term outcomes for islet-alone and islet-after-kidney transplantation in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia.
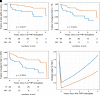
Research design and methods: This was a prospective interventional and observational cohort study of islet-alone (n = 48) and islet-after-kidney (n = 24) transplant recipients followed for up to 8 years after intraportal infusion of one or more purified human pancreatic islet products under standardized immunosuppression. Outcomes included duration of islet graft survival (stimulated C-peptide ≥0.3 ng/mL), on-target glycemic control (HbA1c <7.0%), freedom from severe hypoglycemia, and insulin independence.
Results: Of the 48 islet-alone and 24 islet-after-kidney transplantation recipients, 26 and 8 completed long-term follow-up with islet graft function, 15 and 7 withdrew from follow-up with islet graft function, and 7 and 9 experienced islet graft failure, respectively. Actuarial islet graft survival at median and final follow-up was 84% and 56% for islet-alone and 69% and 49% for islet-after-kidney (P = 0.007) with 77% and 49% of islet-alone and 57% and 35% of islet-after-kidney transplantation recipients maintaining posttransplant HbA1c <7.0% (P = 0.0017); freedom from severe hypoglycemia was maintained at >90% in both cohorts. Insulin independence was achieved by 74% of islet-alone and islet-after-kidney transplantation recipients, with more than one-half maintaining insulin independence during long-term follow-up. Kidney function remained stable during long-term follow-up in both cohorts, and rates of sensitization against HLA were low. Severe adverse events occurred at 0.31 per patient-year for islet-alone and 0.43 per patient-year for islet-after-kidney transplantation.
Conclusions: Islet transplantation results in durable islet graft survival permitting achievement of glycemic targets in the absence of severe hypoglycemia for most appropriately indicated recipients having impaired awareness of hypoglycemia, with acceptable safety of added immunosuppression for both islet-alone and islet-after-kidney transplantation.
© 2022 by the American Diabetes Association.
Figures


References
Grants and funding
- M01 RR000400/RR/NCRR NIH HHS/United States
- U01 AI089316/AI/NIAID NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- U01 AI065192/AI/NIAID NIH HHS/United States
- UL1 RR025741/RR/NCRR NIH HHS/United States
- U01 DK070460/DK/NIDDK NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- U01 AI065193/AI/NIAID NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK070430/DK/NIDDK NIH HHS/United States
- U01 AI089317/AI/NIAID NIH HHS/United States
- U01 DK085531/DK/NIDDK NIH HHS/United States
- U01 DK070431/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- U01 AI065191/AI/NIAID NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR000460/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

