Evidence for a fragile X messenger ribonucleoprotein 1 (FMR1) mRNA gain-of-function toxicity mechanism contributing to the pathogenesis of fragile X-associated premature ovarian insufficiency
- PMID: 36250920
- PMCID: PMC9828574
- DOI: 10.1096/fj.202200468RR
Evidence for a fragile X messenger ribonucleoprotein 1 (FMR1) mRNA gain-of-function toxicity mechanism contributing to the pathogenesis of fragile X-associated premature ovarian insufficiency
Abstract
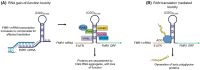
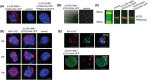
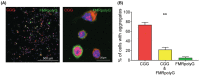
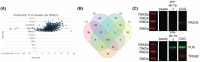
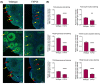
Fragile X-associated premature ovarian insufficiency (FXPOI) is among a family of disorders caused by expansion of a CGG trinucleotide repeat sequence located in the 5' untranslated region (UTR) of the fragile X messenger ribonucleoprotein 1 (FMR1) gene on the X chromosome. Women with FXPOI have a depleted ovarian follicle population, resulting in amenorrhea, hypoestrogenism, and loss of fertility before the age of 40. FXPOI is caused by expansions of the CGG sequence to lengths between 55 and 200 repeats, known as a FMRI premutation, however the mechanism by which the premutation drives disease pathogenesis remains unclear. Two main hypotheses exist, which describe an mRNA toxic gain-of-function mechanism or a protein-based mechanism, where repeat-associated non-AUG (RAN) translation results in the production of an abnormal protein, called FMRpolyG. Here, we have developed an in vitro granulosa cell model of the FMR1 premutation by ectopically expressing CGG-repeat RNA and FMRpolyG protein. We show that expanded CGG-repeat RNA accumulated in intranuclear RNA structures, and these aggregates were able to cause significant granulosa cell death independent of FMRpolyG expression. Using an innovative RNA pulldown, mass spectrometry-based approach we have identified proteins that are specifically sequestered by CGG RNA aggregates in granulosa cells in vitro, and thus may be deregulated as consequence of this interaction. Furthermore, we have demonstrated reduced expression of three proteins identified via our RNA pulldown (FUS, PA2G4 and TRA2β) in ovarian follicles in a FMR1 premutation mouse model. Collectively, these data provide evidence for the contribution of an mRNA gain-of-function mechanism to FXPOI disease biology.
Keywords: CGG trinucleotide repeats; FMRpolyG; FXPOI; mRNA gain-of-function.
© 2022 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures


References
-
- Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38:269‐274. - PubMed
-
- Conway GS, Payne NN, Webb J, Murray A, Jacobs PA. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod. 1998;13:1184‐1187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

