Arylboration of Enecarbamates for the Synthesis of Borylated Saturated N-Heterocycles
- PMID: 36250954
- PMCID: PMC9643676
- DOI: 10.1002/anie.202212117
Arylboration of Enecarbamates for the Synthesis of Borylated Saturated N-Heterocycles
Abstract
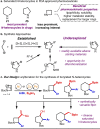
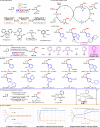
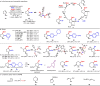
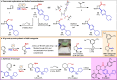
Two catalytic systems have been developed for the arylboration of endocyclic enecarbamates to deliver synthetically versatile borylated saturated N-heterocycles in good regio- and diastereoselectivities. A Cu/Pd dual catalytic reaction enables the synthesis of borylated, α-arylated azetidines, while a Ni-catalysed arylboration reaction efficiently functionalizes 5-, 6-, and 7-membered enecarbamates. In the case of the Cu/Pd-system, a remarkable additive effect was identified that allowed for broader scope. The products are synthetically useful, as demonstrated by manipulations of the boronic ester to access biologically active compounds.
Keywords: Alkene; Arylation; Boron; Cross Coupling; Heterocycles.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274. - PubMed
-
- Wakana D., Kawahara N., Goda Y., Chem. Pharm. Bull. 2013, 61, 1315–1317. - PubMed
-
- None
-
- Brown A., Brown T. B., Calabrese A., Ellis D., Puhalo N., Ralph M., Watson L., Bioorg. Med. Chem. Lett. 2010, 20, 516–520; - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

