Population pharmacokinetics modeling and exposure-response analyses of cemiplimab in patients with recurrent or metastatic cervical cancer
- PMID: 36251220
- PMCID: PMC9662200
- DOI: 10.1002/psp4.12855
Population pharmacokinetics modeling and exposure-response analyses of cemiplimab in patients with recurrent or metastatic cervical cancer
Abstract
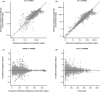
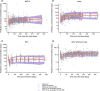
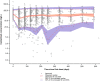
A population pharmacokinetic (PopPK) model was previously developed for cemiplimab in patients with solid tumors, including advanced cutaneous squamous cell carcinoma (CSCC). Here, we update the existing PopPK model and characterize exposure-response relationships using efficacy and safety data obtained in patients with recurrent or metastatic cervical cancer (R/M CC). To improve model stability and robustness of the existing PopPK model in 1062 patients, the random-effect error model was revised, and structural covariates were removed from the base model to be tested in the covariate analysis. The updated model was used for external validation of cemiplimab pharmacokinetics (PK) in patients with R/M CC on cemiplimab monotherapy (350 mg every 3 weeks intravenously) from a phase III study (NCT03257267). Exposure-response relationships for cemiplimab efficacy (overall survival [OS], progression-free survival [PFS], duration of response [DOR], objective response rate [ORR]), and safety (immune-related adverse events [irAEs]) were analyzed in 295 patients with R/M CC from the aforementioned study. The updated PopPK model showed improved stability with 94.8% successful bootstrap runs vs. 47.6% in the prior model. Cemiplimab exposure was similar across tumor types, including basal cell carcinoma, CSCC, and non-small cell lung cancer. External validation showed the updated model adequately described cemiplimab PK in patients with R/M CC. In exposure-response efficacy analyses, Cox proportional hazard modeling (CPHM) showed no trend between exposure and OS, Kaplan-Meier plots showed no trend between exposure and PFS or DOR, and logistic regression analyses conducted on ORR showed no exposure-response relationship. In exposure-response safety analyses, CPHM showed no trend between exposure and irAEs.
© 2022 Regeneron Pharmaceuticals, Inc. CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.H.N., A.P., D.C., and J.D.D. are employees of and stockholders in Regeneron Pharmaceuticals, Inc. D.E., N.D., and N.A.‐H. are full‐time employees of the Ann Arbor Pharmacometrics Group (A2PG) and consultants for Regeneron Pharmaceuticals, Inc.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in oncology: cervical cancer (version 1.2022). 2021. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. Accessed November 20, 2021.

