Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial
- PMID: 36251300
- PMCID: PMC9577883
- DOI: 10.1001/jamaneurol.2022.3392
Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial
Abstract
Importance: Plasma biomarkers of Alzheimer disease may be useful as minimally invasive pharmacodynamic measures of treatment outcomes.
Objective: To analyze the association of donanemab treatment with plasma biomarkers associated with Alzheimer disease.
Design, setting, and participants: TRAILBLAZER-ALZ was a randomized, double-blind, placebo-controlled clinical trial conducted from December 18, 2017, to December 4, 2020, across 56 sites in the US and Canada. Exploratory biomarkers were prespecified with the post hoc addition of plasma glial fibrillary acidic protein and amyloid-β. Men and women aged 60 to 85 years with gradual and progressive change in memory function for at least 6 months were included. A total of 1955 participants were assessed for eligibility. Key eligibility criteria include Mini-Mental State Examination scores of 20 to 28 and elevated amyloid and intermediate tau levels.
Interventions: Randomized participants received donanemab or placebo every 4 weeks for up to 72 weeks. The first 3 doses of donanemab were given at 700 mg and then increased to 1400 mg with blinded dose reductions as specified based on amyloid reduction.
Main outcomes and measures: Change in plasma biomarker levels after donanemab treatment.
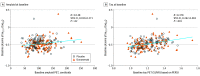
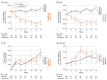
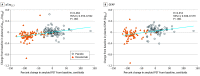
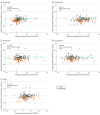
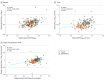
Results: In TRAILBLAZER-ALZ, 272 participants (mean [SD] age, 75.2 [5.5] years; 145 [53.3%] female) were randomized. Plasma levels of phosphorylated tau217 (pTau217) and glial fibrillary acidic protein were significantly lower with donanemab treatment compared with placebo as early as 12 weeks after the start of treatment (least square mean change difference vs placebo, -0.04 [95% CI, -0.07 to -0.02]; P = .002 and -0.04 [95% CI, -0.07 to -0.01]; P = .01, respectively). No significant differences in plasma levels of amyloid-β 42/40 and neurofilament light chain were observed between treatment arms at the end of treatment. Changes in plasma pTau217 and glial fibrillary acidic protein were significantly correlated with the Centiloid percent change in amyloid (Spearman rank correlation coefficient [R] = 0.484 [95% CI, 0.359-0.592]; P < .001 and R = 0.453 [95% CI, 0.306-0.579]; P < .001, respectively) following treatment. Additionally, plasma levels of pTau217 and glial fibrillary acidic protein were significantly correlated at baseline and following treatment (R = 0.399 [95% CI, 0.278-0.508], P < .001 and R = 0.393 [95% CI, 0.254-0.517]; P < .001, respectively).
Conclusions and relevance: Significant reductions in plasma biomarkers pTau217 and glial fibrillary acidic protein compared with placebo were observed following donanemab treatment in patients with early symptomatic Alzheimer disease. These easily accessible plasma biomarkers might provide additional evidence of Alzheimer disease pathology change through anti-amyloid therapy. Usefulness in assessing treatment response will require further evaluation.
Trial registration: ClinicalTrials.gov Identifier: NCT03367403.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

