Radical SAM enzymes: Nature's choice for radical reactions
- PMID: 36251330
- PMCID: PMC9894703
- DOI: 10.1002/1873-3468.14519
Radical SAM enzymes: Nature's choice for radical reactions
Abstract
Enzymes that use a [4Fe-4S]1+ cluster plus S-adenosyl-l-methionine (SAM) to initiate radical reactions (radical SAM) form the largest enzyme superfamily, with over half a million members across the tree of life. This review summarizes recent work revealing the radical SAM reaction pathway, which ultimately liberates the 5'-deoxyadenosyl (5'-dAdo•) radical to perform extremely diverse, highly regio- and stereo-specific, transformations. Most surprising was the discovery of an organometallic intermediate Ω exhibiting an Fe-C5'-adenosyl bond. Ω liberates 5'-dAdo• through homolysis of the Fe-C5' bond, in analogy to Co-C5' bond homolysis in B12 , previously viewed as biology's paradigmatic radical generator. The 5'-dAdo• has been trapped and characterized in radical SAM enzymes via a recently discovered photoreactivity of the [4Fe-4S]+ /SAM complex, and has been confirmed as a catalytically active intermediate in enzyme catalysis. The regioselective SAM S-C bond cleavage to produce 5'-dAdo• originates in the Jahn-Teller effect. The simplicity of SAM as a radical precursor, and the exquisite control of 5'-dAdo• reactivity in radical SAM enzymes, may be why radical SAM enzymes pervade the tree of life, while B12 enzymes are only a few.
Keywords: B12; S-adenosylmethionine; adenosylcobalamin; deoxyadenosyl radical; electron paramagnetic resonance; mechanism; radical; radical SAM.
© 2022 Federation of European Biochemical Societies.
Figures




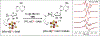

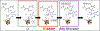
Similar articles
-
ENDOR Spectroscopy Reveals the "Free" 5'-Deoxyadenosyl Radical in a Radical SAM Enzyme Active Site Actually is Chaperoned by Close Interaction with the Methionine-Bound [4Fe-4S]2+ Cluster.J Am Chem Soc. 2024 Feb 14;146(6):3710-3720. doi: 10.1021/jacs.3c09428. Epub 2024 Feb 3. J Am Chem Soc. 2024. PMID: 38308759 Free PMC article.
-
Mechanism of Radical Initiation in the Radical SAM Enzyme Superfamily.Annu Rev Biochem. 2023 Jun 20;92:333-349. doi: 10.1146/annurev-biochem-052621-090638. Epub 2023 Apr 4. Annu Rev Biochem. 2023. PMID: 37018846 Free PMC article. Review.
-
Mechanism of Radical Initiation in the Radical S-Adenosyl-l-methionine Superfamily.Acc Chem Res. 2018 Nov 20;51(11):2611-2619. doi: 10.1021/acs.accounts.8b00356. Epub 2018 Oct 15. Acc Chem Res. 2018. PMID: 30346729 Free PMC article. Review.
-
Paradigm Shift for Radical S-Adenosyl-l-methionine Reactions: The Organometallic Intermediate Ω Is Central to Catalysis.J Am Chem Soc. 2018 Jul 18;140(28):8634-8638. doi: 10.1021/jacs.8b04061. Epub 2018 Jul 6. J Am Chem Soc. 2018. PMID: 29954180 Free PMC article.
-
Radical SAM Enzyme Spore Photoproduct Lyase: Properties of the Ω Organometallic Intermediate and Identification of Stable Protein Radicals Formed during Substrate-Free Turnover.J Am Chem Soc. 2020 Oct 28;142(43):18652-18660. doi: 10.1021/jacs.0c08585. Epub 2020 Oct 15. J Am Chem Soc. 2020. PMID: 32966073 Free PMC article.
Cited by
-
Iron-sulfur protein odyssey: exploring their cluster functional versatility and challenging identification.Metallomics. 2024 May 2;16(5):mfae025. doi: 10.1093/mtomcs/mfae025. Metallomics. 2024. PMID: 38744662 Free PMC article. Review.
-
Stable organic radical qubits and their applications in quantum information science.Innovation (Camb). 2024 Jun 21;5(5):100662. doi: 10.1016/j.xinn.2024.100662. eCollection 2024 Sep 9. Innovation (Camb). 2024. PMID: 39091459 Free PMC article. Review.
-
Sulfide regulation and catabolism in health and disease.Signal Transduct Target Ther. 2025 May 30;10(1):174. doi: 10.1038/s41392-025-02231-w. Signal Transduct Target Ther. 2025. PMID: 40442106 Free PMC article. Review.
-
Aromatic side-chain crosslinking in RiPP biosynthesis.Nat Chem Biol. 2025 Feb;21(2):168-181. doi: 10.1038/s41589-024-01795-y. Epub 2025 Jan 15. Nat Chem Biol. 2025. PMID: 39814993 Free PMC article. Review.
-
ENDOR Spectroscopy Reveals the "Free" 5'-Deoxyadenosyl Radical in a Radical SAM Enzyme Active Site Actually is Chaperoned by Close Interaction with the Methionine-Bound [4Fe-4S]2+ Cluster.J Am Chem Soc. 2024 Feb 14;146(6):3710-3720. doi: 10.1021/jacs.3c09428. Epub 2024 Feb 3. J Am Chem Soc. 2024. PMID: 38308759 Free PMC article.
References
-
- Pryor WA (1978) The formation of free radicals and the consequences of their reactions in vivo, Photochem Photobiol. 28, 787–797. - PubMed
-
- Williams RJP (1985) The necessary and the desirable production of radicals in biology, Phil Trans R Soc Lond B. 311, 593–603. - PubMed
-
- Stubbe J.(1988) Radicals in Biological Catalysis, Biochemistry. 27, 3893–3900. - PubMed
-
- Giese B.(1986) Radicals in organic synthesis: Formation of carbon-carbon bonds, Pergamom, Elmsford, NY.
-
- Stubbe J.(1989) Protein radical involvement in biological catalysis?, Annu Rev Biochem. 58, 257–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

