Bn2DT3A, a Chelator for 68Ga Positron Emission Tomography: Hydroxide Coordination Increases Biological Stability of [68Ga][Ga(Bn2DT3A)(OH)]
- PMID: 36251390
- PMCID: PMC9627565
- DOI: 10.1021/acs.inorgchem.2c01992
Bn2DT3A, a Chelator for 68Ga Positron Emission Tomography: Hydroxide Coordination Increases Biological Stability of [68Ga][Ga(Bn2DT3A)(OH)]
Abstract
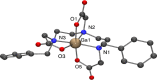
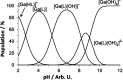
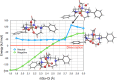
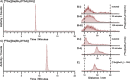
The chelator Bn2DT3A was used to produce a novel 68Ga complex for positron emission tomography (PET). Unusually, this system is stabilized by a coordinated hydroxide in aqueous solutions above pH 5, which confers sufficient stability for it to be used for PET. Bn2DT3A complexes Ga3+ in a hexadentate manner, forming a mer-mer complex with log K([Ga(Bn2DT3A)]) = 18.25. Above pH 5, the hydroxide ion coordinates the Ga3+ ion following dissociation of a coordinated amine. Bn2DT3A radiolabeling displayed a pH-dependent speciation, with [68Ga][Ga(Bn2DT3A)(OH)]- being formed above pH 5 and efficiently radiolabeled at pH 7.4. Surprisingly, [68Ga][Ga(Bn2DT3A)(OH)]- was found to show an increased stability in vitro (for over 2 h in fetal bovine serum) compared to [68Ga][Ga(Bn2DT3A)]. The biodistribution of [68Ga][Ga(Bn2DT3A)(OH)]- in healthy rats showed rapid clearance and excretion via the kidneys, with no uptake seen in the lungs or bones.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Blower J. E.; Cooper M. S.; Imberti C.; Ma M. T.; Marshall C.; Young J. D.; Blower P. J.. The Radiopharmaceutical Chemistry of the Radionuclides of Gallium and Indium. In Radiopharmaceutical Chemistry; Lewis J. S., Windhorst A. D., Zeglis B. M., Eds.; Springer, Cham: Cham, 2019; pp. 255–271, 10.1007/978-3-319-98947-1_14. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

