The measurement of autoantibodies to insulin informs diagnosis of diabetes in a childhood population negative for other autoantibodies
- PMID: 36251483
- PMCID: PMC9827938
- DOI: 10.1111/dme.14979
The measurement of autoantibodies to insulin informs diagnosis of diabetes in a childhood population negative for other autoantibodies
Abstract
Aims: Some childhood type 1 diabetes cases are islet autoantibody negative at diagnosis. Potential explanations include misdiagnosis of genetic forms of diabetes or insufficient islet autoantibody testing. Many NHS laboratories offer combinations of three autoantibody markers. We sought to determine the benefit of testing for additional islet autoantibodies, including insulin (IAA) and tetraspanin 7 (TSPAN7A).
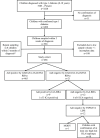
Methods: Radiobinding assays (RBAs) were used to test for four islet autoantibodies in children with newly diagnosed type 1 diabetes (n = 486; 54.1% male; median age 10.4 years [range 0.7-18.0]; median duration 1 day [range -183 to 14]). Islet autoantibody negative children were tested for TSPAN7A using a luminescence-based test. Where available, islet cell antibody (ICA) and human leucocyte antigen (HLA) data were considered.
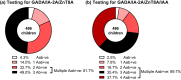
Results: Using three autoantibody markers, 21/486 (4.3%) children were autoantibody negative. Testing for IAA classified a further 9/21 (42.9%) children as autoantibody positive. Of the remaining 12 (2.5%) autoantibody negative children, all were TPAN7A negative, seven were ICA negative and one was positive for the protective variant DQB1*0602. One was subsequently diagnosed with Maturity Onset of Diabetes in the Young, but follow-up was not available in all cases.
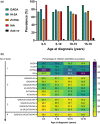
Conclusions: Using highly sensitive assays, testing for three autoantibodies fails to detect islet autoimmunity in approximately 1/20 children diagnosed with type 1 diabetes. Testing for IAA in children <5 years and GADA in those >10 years was the most effective strategy for detecting islet autoimmunity. The ability to test for all islet autoantibodies should inform clinical decisions and make screening for monogenic diabetes more cost-effective.
Keywords: autoimmunity; islet autoantibodies; type 1 diabetes.
© 2022 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures
References
-
- Bottazzo GF, Florin‐Christensen A, Doniach D. Islet‐cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2(7892):1279‐1283. - PubMed
-
- Doniach D, Bottazzo GF. Autoimmunity and the endocrine pancreas. Pathobiol Annu. 1977;7:327‐346. - PubMed
-
- (NICE) NIfHaCE . Diabetes (type 1 and type 2) in children and young people: diagnosis and management NICE guideline [NG18]. 2022. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

