UBR2 targets myosin heavy chain IIb and IIx for degradation: Molecular mechanism essential for cancer-induced muscle wasting
- PMID: 36252004
- PMCID: PMC9618047
- DOI: 10.1073/pnas.2200215119
UBR2 targets myosin heavy chain IIb and IIx for degradation: Molecular mechanism essential for cancer-induced muscle wasting
Abstract
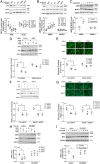
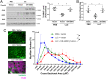
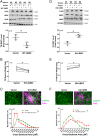
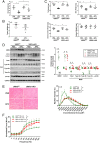
Cancer cachexia is a lethal metabolic syndrome featuring muscle wasting with preferential loss of fast-twitching muscle mass through an undefined mechanism. Here, we show that cancer induces muscle wasting by selectively degrading myosin heavy chain (MHC) subtypes IIb and IIx through E3 ligase UBR2-mediated ubiquitylation. Induction of MHC loss and atrophy in C2C12 myotubes and mouse tibialis anterior (TA) by murine cancer cells required UBR2 up-regulation by cancer. Genetic gain or loss of UBR2 function inversely altered MHC level and muscle mass in TA of tumor-free mice. UBR2 selectively interacted with and ubiquitylated MHC-IIb and MHC-IIx through its substrate recognition and catalytic domain, respectively, in C2C12 myotubes. Elevation of UBR2 in muscle of tumor-bearing or free mice caused loss of MHC-IIb and MHC-IIx but not MHC-I and MHC-IIa or other myofibrillar proteins, including α-actin, troponin, tropomyosin, and tropomodulin. Muscle-specific knockout of UBR2 spared KPC tumor-bearing mice from losing MHC-IIb and MHC-IIx, fast-twitching muscle mass, cross-sectional area, and contractile force. The rectus abdominis (RA) muscle of patients with cachexia-prone cancers displayed a selective reduction of MHC-IIx in correlation with higher UBR2 levels. These data suggest that UBR2 is a regulator of MHC-IIb/IIx essential for cancer-induced muscle wasting, and that therapeutic interventions can be designed by blocking UBR2 up-regulation by cancer.
Keywords: MHC-IIb; MHC-IIx; UBR2; cancer cachexia; ubiquitylation.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Sadeghi M., et al. , Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 127, 91–104 (2018). - PubMed
-
- Fearon K., et al. , Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 12, 489–495 (2011). - PubMed
-
- Acharyya S., et al. , Dystrophin glycoprotein complex dysfunction: A regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell 8, 421–432 (2005). - PubMed
-
- Li Y. P., Schwartz R. J., Waddell I. D., Holloway B. R., Reid M. B., Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 12, 871–880 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

