Wettability-based ultrasensitive detection of amphiphiles through directed concentration at disordered regions in self-assembled monolayers
- PMID: 36252006
- PMCID: PMC9618133
- DOI: 10.1073/pnas.2211042119
Wettability-based ultrasensitive detection of amphiphiles through directed concentration at disordered regions in self-assembled monolayers
Abstract
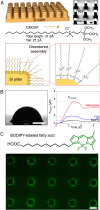
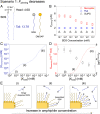
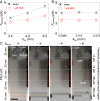
Various forms of ecological monitoring and disease diagnosis rely upon the detection of amphiphiles, including lipids, lipopolysaccharides, and lipoproteins, at ultralow concentrations in small droplets. Although assays based on droplets' wettability provide promising options in some cases, their reliance on the measurements of surface and bulk properties of whole droplets (e.g., contact angles, surface tensions) makes it difficult to monitor trace amounts of these amphiphiles within small-volume samples. Here, we report a design principle in which self-assembled monolayer-functionalized microstructured surfaces coated with silicone oil create locally disordered regions within a droplet's contact lines to effectively concentrate amphiphiles within the areas that dominate the droplet static friction. Remarkably, such surfaces enable the ultrasensitive, naked-eye detection of amphiphiles through changes in the droplets' sliding angles, even when the concentration is four to five orders of magnitude below their critical micelle concentration. We develop a thermodynamic model to explain the partitioning of amphiphiles at the contact line by their cooperative association within the disordered, loosely packed regions of the self-assembled monolayer. Based on this local analyte concentrating effect, we showcase laboratory-on-a-chip surfaces with positionally dependent pinning forces capable of both detecting industrially and biologically relevant amphiphiles (e.g., bacterial endotoxins), as well as sorting aqueous droplets into discrete groups based on their amphiphile concentrations. Furthermore, we demonstrate that the sliding behavior of amphiphile-laden aqueous droplets provides insight into the amphiphile's effective length, thereby allowing these surfaces to discriminate between analytes with highly disparate molecular sizes.
Keywords: amphiphiles; lubricated surfaces; self-assembly; sensors; wettability.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Liquid Crystal-Infused Porous Polymer Surfaces: A "Slippery" Soft Material Platform for the Naked-Eye Detection and Discrimination of Amphiphilic Species.ACS Appl Mater Interfaces. 2021 Jul 21;13(28):33652-33663. doi: 10.1021/acsami.1c08170. Epub 2021 Jul 8. ACS Appl Mater Interfaces. 2021. PMID: 34236833 Free PMC article.
-
Large organized surface domains self-assembled from nonpolar amphiphiles.Acc Chem Res. 2012 Apr 17;45(4):514-24. doi: 10.1021/ar200178a. Epub 2011 Dec 21. Acc Chem Res. 2012. PMID: 22185721 Review.
-
Effect of siloxane spacer length on organosilicon bi-quaternary ammonium amphiphiles.Colloids Surf B Biointerfaces. 2015 Apr 1;128:528-536. doi: 10.1016/j.colsurfb.2015.03.004. Epub 2015 Mar 7. Colloids Surf B Biointerfaces. 2015. PMID: 25794442
-
Surfactant solutions and porous substrates: spreading and imbibition.Adv Colloid Interface Sci. 2004 Nov 29;111(1-2):3-27. doi: 10.1016/j.cis.2004.07.007. Adv Colloid Interface Sci. 2004. PMID: 15571660
-
Phases and phase transition in insoluble and adsorbed monolayers of amide amphiphiles: Specific characteristics of the condensed phases.Adv Colloid Interface Sci. 2015 Aug;222:728-42. doi: 10.1016/j.cis.2014.07.014. Epub 2014 Aug 6. Adv Colloid Interface Sci. 2015. PMID: 25129816 Review.
Cited by
-
Self-Assembled Monolayers of Push-Pull Chromophores as Active Layers and Their Applications.Molecules. 2024 Jan 23;29(3):559. doi: 10.3390/molecules29030559. Molecules. 2024. PMID: 38338304 Free PMC article. Review.
-
Synergistic Adhesion and Shape Deformation in Nanowire-Structured Liquid Crystal Elastomers.Adv Mater. 2025 Mar;37(9):e2414695. doi: 10.1002/adma.202414695. Epub 2025 Jan 19. Adv Mater. 2025. PMID: 39828612 Free PMC article.
-
Precise Oligomer Organization Enhanced Electrostatic Interactions for Efficient Cell Membrane Binding.Nano Lett. 2025 May 28;25(21):8488-8494. doi: 10.1021/acs.nanolett.5c00651. Epub 2025 May 16. Nano Lett. 2025. PMID: 40377435 Free PMC article.
-
A phenothiazine-functionalized pyridine-based AIEE-active molecule: a versatile molecular probe for highly sensitive detection of hypochlorite and picric acid.RSC Adv. 2024 Feb 8;14(8):5149-5158. doi: 10.1039/d3ra08451e. eCollection 2024 Feb 7. RSC Adv. 2024. PMID: 38332784 Free PMC article.
-
Visualization and Experimental Characterization of Wrapping Layer Using Planar Laser-Induced Fluorescence.ACS Nano. 2024 Feb 6;18(5):4068-4076. doi: 10.1021/acsnano.3c07407. Epub 2024 Jan 26. ACS Nano. 2024. PMID: 38277478 Free PMC article.
References
-
- Yushmanov V. E., Perussi J. R., Imasato H., Tabak M., Interaction of papaverine with micelles of surfactants with different charge studied by 1H-NMR. Biochim. Biophys. Acta 1189, 74–80 (1994). - PubMed
-
- Olkowska E., Polkowska Ż., Namieśnik J., Analytics of surfactants in the environment: Problems and challenges. Chem. Rev. 111, 5667–5700 (2011). - PubMed
-
- Cserháti T., Forgács E., Oros G., Biological activity and environmental impact of anionic surfactants. Environ. Int. 28, 337–348 (2002). - PubMed
-
- Lechuga M., Fernández-Serrano M., Jurado E., Núñez-Olea J., Ríos F., Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 125, 1–8 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

