A role for axon-glial interactions and Netrin-G1 signaling in the formation of low-threshold mechanoreceptor end organs
- PMID: 36252008
- PMCID: PMC9618144
- DOI: 10.1073/pnas.2210421119
A role for axon-glial interactions and Netrin-G1 signaling in the formation of low-threshold mechanoreceptor end organs
Abstract
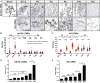
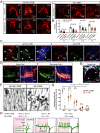
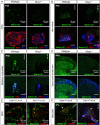
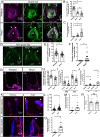
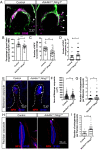
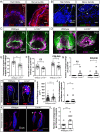
Low-threshold mechanoreceptors (LTMRs) and their cutaneous end organs convert light mechanical forces acting on the skin into electrical signals that propagate to the central nervous system. In mouse hairy skin, hair follicle-associated longitudinal lanceolate complexes, which are end organs comprising LTMR axonal endings that intimately associate with terminal Schwann cell (TSC) processes, mediate LTMR responses to hair deflection and skin indentation. Here, we characterized developmental steps leading to the formation of Aβ rapidly adapting (RA)-LTMR and Aδ-LTMR lanceolate complexes. During early postnatal development, Aβ RA-LTMRs and Aδ-LTMRs extend and prune cutaneous axonal branches in close association with nascent TSC processes. Netrin-G1 is expressed in these developing Aβ RA-LTMR and Aδ-LTMR lanceolate endings, and Ntng1 ablation experiments indicate that Netrin-G1 functions in sensory neurons to promote lanceolate ending elaboration around hair follicles. The Netrin-G ligand (NGL-1), encoded by Lrrc4c, is expressed in TSCs, and ablation of Lrrc4c partially phenocopied the lanceolate complex deficits observed in Ntng1 mutants. Moreover, NGL-1-Netrin-G1 signaling is a general mediator of LTMR end organ formation across diverse tissue types demonstrated by the fact that Aβ RA-LTMR endings associated with Meissner corpuscles and Pacinian corpuscles are also compromised in the Ntng1 and Lrrc4c mutant mice. Thus, axon-glia interactions, mediated in part by NGL-1-Netrin-G1 signaling, promote LTMR end organ formation.
Keywords: Netrin-G1; axon–glia interaction; development; lanceolate complex; pruning.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Three-dimensional reconstructions of mechanosensory end organs suggest a unifying mechanism underlying dynamic, light touch.bioRxiv [Preprint]. 2023 Mar 18:2023.03.17.533188. doi: 10.1101/2023.03.17.533188. bioRxiv. 2023. Update in: Neuron. 2023 Oct 18;111(20):3211-3229.e9. doi: 10.1016/j.neuron.2023.08.023. PMID: 36993253 Free PMC article. Updated. Preprint.
-
The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles.Elife. 2014 Feb 25;3:e01901. doi: 10.7554/eLife.01901. Elife. 2014. PMID: 24569481 Free PMC article.
-
The cellular and molecular basis of direction selectivity of Aδ-LTMRs.Cell. 2014 Dec 18;159(7):1640-51. doi: 10.1016/j.cell.2014.11.038. Cell. 2014. PMID: 25525881 Free PMC article.
-
The mechanosensory neurons of touch and their mechanisms of activation.Nat Rev Neurosci. 2021 Sep;22(9):521-537. doi: 10.1038/s41583-021-00489-x. Epub 2021 Jul 26. Nat Rev Neurosci. 2021. PMID: 34312536 Free PMC article. Review.
-
The gentle touch receptors of mammalian skin.Science. 2014 Nov 21;346(6212):950-4. doi: 10.1126/science.1254229. Science. 2014. PMID: 25414303 Free PMC article. Review.
Cited by
-
The inner core enables transient touch detection in the Pacinian corpuscle.Sci Adv. 2025 Feb 28;11(9):eadt4837. doi: 10.1126/sciadv.adt4837. Epub 2025 Feb 26. Sci Adv. 2025. PMID: 40009676 Free PMC article.
-
Gene expression profiling in pure neural leprosy: insights into pathogenesis and diagnostic biomarkers.Front Immunol. 2025 May 12;16:1550687. doi: 10.3389/fimmu.2025.1550687. eCollection 2025. Front Immunol. 2025. PMID: 40421009 Free PMC article.
-
Structural and functional dissection of the Pacinian corpuscle reveals an active role of the inner core in touch detection.bioRxiv [Preprint]. 2024 Aug 26:2024.08.24.609509. doi: 10.1101/2024.08.24.609509. bioRxiv. 2024. Update in: Sci Adv. 2025 Feb 28;11(9):eadt4837. doi: 10.1126/sciadv.adt4837. PMID: 39253434 Free PMC article. Updated. Preprint.
-
Skin-type-dependent development of murine mechanosensory neurons.Dev Cell. 2023 Oct 23;58(20):2032-2047.e6. doi: 10.1016/j.devcel.2023.07.020. Epub 2023 Aug 21. Dev Cell. 2023. PMID: 37607547 Free PMC article.
-
Exploration of the Core Pathways and Potential Targets of Luteolin Treatment on Late-Onset Depression Based on Cerebrospinal Fluid Proteomics.Int J Mol Sci. 2023 Feb 9;24(4):3485. doi: 10.3390/ijms24043485. Int J Mol Sci. 2023. PMID: 36834894 Free PMC article.
References
-
- Horch K. W., Tuckett R. P., Burgess P. R., A key to the classification of cutaneous mechanoreceptors. J. Invest. Dermatol. 69, 75–82 (1977). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

