The ulcerative colitis-associated gene FUT8 regulates the quantity and quality of secreted mucins
- PMID: 36252012
- PMCID: PMC9618082
- DOI: 10.1073/pnas.2205277119
The ulcerative colitis-associated gene FUT8 regulates the quantity and quality of secreted mucins
Abstract
Mucins are the main macrocomponents of the mucus layer that protects the digestive tract from pathogens. Fucosylation of mucins increases mucus viscoelasticity and its resistance to shear stress. These properties are altered in patients with ulcerative colitis (UC), which is marked by a chronic inflammation of the distal part of the colon. Here, we show that levels of Fucosyltransferase 8 (FUT8) and specific mucins are increased in the distal inflamed colon of UC patients. Recapitulating this FUT8 overexpression in mucin-producing HT29-18N2 colonic cell line increases delivery of MUC1 to the plasma membrane and extracellular release of MUC2 and MUC5AC. Mucins secreted by FUT8 overexpressing cells are more resistant to removal from the cell surface than mucins secreted by FUT8-depleted cells (FUT8 KD). FUT8 KD causes intracellular accumulation of MUC1 and alters the ratio of secreted MUC2 to MUC5AC. These data fit well with the Fut8-/- mice phenotype, which are protected from UC. Fut8-/- mice exhibit a thinner proximal colon mucus layer with an altered ratio of neutral to acidic mucins. Together, our data reveal that FUT8 modifies the biophysical properties of mucus by controlling levels of cell surface MUC1 and quantity and quality of secreted MUC2 and MUC5AC. We suggest that these changes in mucus viscoelasticity likely facilitate bacterial-epithelial interactions leading to inflammation and UC progression.
Keywords: FUT8; fucosylation; mucin secretion; ulcerative colitis.
Conflict of interest statement
The authors declare no competing interest.
Figures
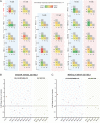

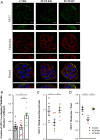



References
-
- Thornton D. J., Rousseau K., McGuckin M. A., Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

