14-3-3τ drives estrogen receptor loss via ERα36 induction and GATA3 inhibition in breast cancer
- PMID: 36252018
- PMCID: PMC9618134
- DOI: 10.1073/pnas.2209211119
14-3-3τ drives estrogen receptor loss via ERα36 induction and GATA3 inhibition in breast cancer
Abstract
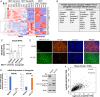
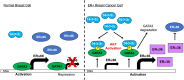
About one-fourth of recurrent estrogen receptor-positive (ER+) breast cancers lose ER expression, leading to endocrine therapy failure. However, the mechanisms underlying ER loss remain to be fully explored. We now show that 14-3-3τ, up-regulated in ∼60% of breast cancer, drives the conversion of ER+ to ER- and epithelial-to-mesenchymal transition (EMT). We identify ERα36, an isoform of ERα66, as a downstream effector of 14-3-3τ. Overexpression of 14-3-3τ induces ERα36 in xenografts and tumor spheroids. The regulation is further supported by a positive correlation between ERα36 and 14-3-3τ expression in human breast cancers. ERα36 can antagonize ERα66 and inhibit ERα66 expression. Isoform-specific depletion of ERα36 blocks the ER conversion and EMT induced by 14-3-3τ overexpression in tumor spheroids, thus establishing ERα36 as a key mediator in 14-3-3τ-driven ER loss and EMT. ERα36 promoter is repressed by GATA3, which can be phosphorylated by AKT at consensus binding sites for 14-3-3. Upon AKT activation, 14-3-3τ binds phosphorylated GATA3 and facilitates the degradation of GATA3 causing GATA3 to lose transcriptional control over its target genes ERα66 and ERα36. We also demonstrate a role for the collaboration between 14-3-3τ and AKT in ERα36 induction and endocrine therapy resistance by three-dimensional spheroid and tamoxifen treatment models in MCF7 and T47D ER+ breast cancer cells. Thus, the 14-3-3τ-ERα36 regulation provides a previously unrecognized mechanism for ER loss and endocrine therapy failure.
Keywords: 14-3-3τ; 3D tumor spheroid model; ERα36; GATA3; estrogen receptor.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Burstein H. J., et al. , Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J. Clin. Oncol. 37, 423–438 (2019). - PubMed
-
- Davies C., et al. ; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group, Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381, 805–816 (2013). - PMC - PubMed
-
- Aurilio G., et al. , A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur. J. Cancer 50, 277–289 (2014). - PubMed
-
- Wang Z. Y., Yin L., Estrogen receptor alpha-36 (ER-α36): A new player in human breast cancer. Mol. Cell. Endocrinol. 418, 193–206 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

