Dynamic processing of hunger and thirst by common mesolimbic neural ensembles
- PMID: 36252036
- PMCID: PMC9618039
- DOI: 10.1073/pnas.2211688119
Dynamic processing of hunger and thirst by common mesolimbic neural ensembles
Abstract
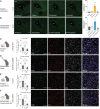
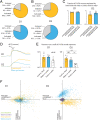
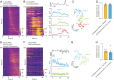
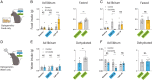
The nucleus accumbens (NAc) is a canonical reward center that regulates feeding and drinking but it is not known whether these behaviors are mediated by same or different neurons. We employed two-photon calcium imaging in awake, behaving mice and found that during the appetitive phase, both hunger and thirst are sensed by a nearly identical population of individual D1 and D2 neurons in the NAc that respond monophasically to food cues in fasted animals and water cues in dehydrated animals. During the consummatory phase, we identified three distinct neuronal clusters that are temporally correlated with action initiation, consumption, and cessation shared by feeding and drinking. These dynamic clusters also show a nearly complete overlap of individual D1 neurons and extensive overlap among D2 neurons. Modulating D1 and D2 neural activities revealed analogous effects on feeding versus drinking behaviors. In aggregate, these data show that a highly overlapping set of D1 and D2 neurons in NAc detect food and water reward and elicit concordant responses to hunger and thirst. These studies establish a general role of this mesolimbic pathway in mediating instinctive behaviors by controlling motivation-associated variables rather than conferring behavioral specificity.
Keywords: feeding behavior; motivation; need states; reward; two-photon calcium imaging.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- Phillips P. E., Stuber G. D., Heien M. L., Wightman R. M., Carelli R. M., Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

