Identification of Patients With Ovarian Cancer Experiencing the Highest Benefit From Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study
- PMID: 36252167
- PMCID: PMC9746742
- DOI: 10.1200/JCO.22.01207
Identification of Patients With Ovarian Cancer Experiencing the Highest Benefit From Bevacizumab in the First-Line Setting on the Basis of Their Tumor-Intrinsic Chemosensitivity (KELIM): The GOG-0218 Validation Study
Abstract
Purpose: In patients with high-grade ovarian cancer, predictors of bevacizumab efficacy in first-line setting are needed. In the ICON-7 trial, a poor tumor intrinsic chemosensitivity (defined by unfavorable modeled cancer antigen-125 [CA-125] ELIMination rate constant K [KELIM] score) was a predictive biomarker. Only the patients with high-risk disease (suboptimally resected stage III, or stage IV) exhibiting unfavorable KELIM score < 1.0 had overall survival (OS) benefit from bevacizumab (median: 29.7 v 20.6 months; hazard ratio [HR], 0.78). An external validation study in the GOG-0218 trial was performed.
Methods: In GOG-0218, 1,873 patients were treated with carboplatin-paclitaxel ± concurrent-maintenance bevacizumab/placebo. Patient KELIM values were calculated with CA-125 kinetics during the first 100 chemotherapy days by the Lyon University team. The association between KELIM score (favorable ≥ 1.0, or unfavorable < 1.0) and bevacizumab benefit for progression-free survival (PFS)/OS was independently assessed by NGR-GOG using univariate/multivariate analyses.
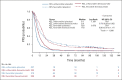
Results: KELIM was assessable in 1,662 patients with ≥ 3 CA-125 available values. An unfavorable KELIM score was associated with bevacizumab benefit compared with placebo (PFS: HR, 0.70; 95% CI, 0.59 to 0.82; OS: HR, 0.87; 95% CI, 0.73 to 1.03), whereas a favorable KELIM was not (PFS: HR, 0.96; 95% CI, 0.79 to 1.17; OS: HR, 1.11; 95% CI, 0.89 to 1.39). The highest benefit was observed in patients with a high-risk disease exhibiting unfavorable KELIM, for PFS (median: 9.1 v 5.6 months; HR, 0.64; 95% CI, 0.53 to 0.78), and for OS (median: 35.1 v 29.1 months; HR, 0.79; 95% CI, 0.65 to 0.97).
Conclusion: This GOG-0218 trial investigation validates ICON-7 findings about the association between poor tumor chemosensitivity and benefit from concurrent-maintenance bevacizumab, suggesting that bevacizumab may mainly be effective in patients with poorly chemosensitive disease. Bevacizumab may be prioritized in patients with a high-risk and poorly chemosensitive disease to improve their PFS/OS (patient KELIM score calculator available on the Biomarker Kinetics website).
Trial registration: ClinicalTrials.gov NCT00262847.
Figures



Comment in
-
Among patients with advanced ovarian carcinoma, who benefits from bevacizumab the most?Ann Transl Med. 2023 Aug 30;11(10):367. doi: 10.21037/atm-23-903. Epub 2023 Mar 28. Ann Transl Med. 2023. PMID: 37675330 Free PMC article. No abstract available.
References
-
- US Food and Drug Administration: FDA approves bevacizumab in combination with chemotherapy for ovarian cancer. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro...
-
- Burger RA, Brady MF, Bookman MA, et al. : Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365:2473-2483, 2011 - PubMed
-
- Perren TJ, Swart AM, Pfisterer J, et al. : A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365:2484-2496, 2011 - PubMed
-
- Colombo N, Sessa C, Bois AD, et al. : ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 30:672-705, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

