The mouse allantois: new insights at the embryonic-extraembryonic interface
- PMID: 36252214
- PMCID: PMC9574631
- DOI: 10.1098/rstb.2021.0251
The mouse allantois: new insights at the embryonic-extraembryonic interface
Abstract
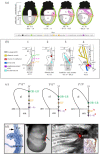
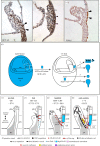
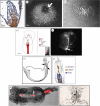
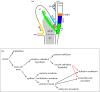
During the early development of Placentalia, a distinctive projection emerges at the posterior embryonic-extraembryonic interface of the conceptus; its fingerlike shape presages maturation into the placental umbilical cord, whose major role is to shuttle fetal blood to and from the chorion for exchange with the mother during pregnancy. Until recently, the biology of the cord's vital vascular anlage, called the body stalk/allantois in humans and simply the allantois in rodents, has been largely unknown. Here, new insights into the development of the mouse allantois are featured, from its origin and mechanism of arterial patterning through its union with the chorion. Key to generating the allantois and its critical functions are the primitive streak and visceral endoderm, which together are sufficient to create the entire fetal-placental connection. Their newly discovered roles at the embryonic-extraembryonic interface challenge conventional wisdom, including the physical limits of the primitive streak, its function as sole purveyor of mesoderm in the mouse, potency of visceral endoderm, and the putative role of the allantois in the germ line. With this working model of allantois development, understanding a plethora of hitherto poorly understood orphan diseases in humans is now within reach. This article is part of the theme issue 'Extraembryonic tissues: exploring concepts, definitions and functions across the animal kingdom'.
Keywords: allantois; arteries; mesoderm; primitive streak; primordial germ cells; visceral endoderm.
Figures

References
-
- Duval M. 1891. Le placenta des rongeurs. Trosième partie. Le placenta de la souris et du rat. J. Anat. Physiol. Normales et Pathol. de l'Homme et des Animaux 27, 24-73, 344–395. 515–612.
-
- Duval M. 1892. Le placenta des rongeurs. In Extrait du journal de l'anatomie et de la physiologie annees 1889–1892 (ed. Alcan F). Paris, France: Ancienne Librarie Gemner Bailliere.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
