Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: recent advances, emerging technologies, and development challenges
- PMID: 36252277
- PMCID: PMC9639748
- DOI: 10.1080/17425247.2022.2135699
Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: recent advances, emerging technologies, and development challenges
Abstract
Introduction: Poor or inconsistent adherence to daily oral pre-exposure prophylaxis (PrEP) has emerged as a key barrier to effective HIV prevention. The advent of potent long-acting (LA) antiretrovirals (ARVs) in conjunction with advances in controlled release technologies has enabled LA ARV drug delivery systems (DDS) capable of providing extended dosing intervals and overcome the challenge of suboptimal drug adherence with daily oral dosing.
Areas covered: This review discusses the current state of the LA PrEP field, recent advances, and emerging technologies, including ARV prodrug modifications and new DDS. Technological challenges, knowledge gaps, preclinical testing considerations, and future directions important in the context of clinical translation and implementation of LA HIV PrEP are discussed.
Expert opinion: The HIV prevention field is evolving faster than ever and the bar for developing next-generation LA HIV prevention options continues to rise. The requirements for viable LA PrEP products to be implemented in resource-limited settings are challenging, necessitating proactive consideration and product modifications during the design and testing of promising new candidates. If successfully translated, next-generation LA PrEP that are safe, affordable, highly effective, and accepted by both end-users and key stakeholders will offer significant potential to curb the HIV pandemic.
Keywords: Adherence; HIV prevention; antiretroviral; drug delivery system; implants; injectable; long-acting; microbicide; nanoformulations; prodrug.
Conflict of interest statement
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures


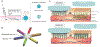
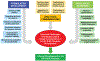
References
-
- UNAIDS. Global HIV & AIDS statistics - fact sheet [Internet]. UNAIDS. 2021. [cited June 28 2022]. cited: https://www.unaids.org/en/resources/fact-sheet.
-
- UNAIDS; Sabin K. The prevention gap report. 2016.
-
- Global AIDS Strategy 2021–2026 — end Inequalities. End AIDS. [Internet] UNAIDS. 2021. [cited Sep 15, 2022]. Available from: https://aidstargets2025.unaids.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
