Importin α/β and the tug of war to keep TDP-43 in solution: quo vadis?
- PMID: 36253101
- PMCID: PMC9969346
- DOI: 10.1002/bies.202200181
Importin α/β and the tug of war to keep TDP-43 in solution: quo vadis?
Abstract
The transactivation response-DNA binding protein of 43 kDa (TDP-43) is an aggregation-prone nucleic acid-binding protein linked to the etiology of Amyotrophic Lateral Sclerosis (ALS) and Frontotemporal Lobar Degeneration (FTLD). These conditions feature the accumulation of insoluble TDP-43 aggregates in the neuronal cytoplasm that lead to cell death. The dynamics between cytoplasmic and nuclear TDP-43 are altered in the disease state where TDP-43 mislocalizes to the cytoplasm, disrupting Nuclear Pore Complexes (NPCs), and ultimately forming large fibrils stabilized by the C-terminal prion-like domain. Here, we review three emerging and poorly understood aspects of TDP-43 biology linked to its aggregation. First, how post-translational modifications in the proximity of TDP-43 N-terminal domain (NTD) promote aggregation. Second, how TDP-43 engages FG-nucleoporins in the NPC, disrupting the pore permeability and function. Third, how the importin α/β heterodimer prevents TDP-43 aggregation, serving both as a nuclear import transporter and a cytoplasmic chaperone.
Keywords: FG-nucleoporins; NTD; TDP-43; neurodegeneration; protein aggregation; importin α/β.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures


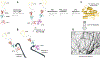
References
-
- Colombrita C, Onesto E, Megiorni F, Pizzuti A, Baralle FE, Buratti E, … Ratti A (2012). TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J Biol Chem, 287(19), 15635–15647. doi: 10.1074/jbc.M111.333450 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

