D-galactose protects the intestine from ionizing radiation-induced injury by altering the gut microbiome
- PMID: 36253108
- PMCID: PMC9726703
- DOI: 10.1093/jrr/rrac059
D-galactose protects the intestine from ionizing radiation-induced injury by altering the gut microbiome
Abstract
This article aims to investigate the protection of the intestine from ionizing radiation-induced injury by using D-galactose (D-gal) to alter the gut microbiome. In addition, this observation opens up further lines of research to further increase therapeutic potentials. Male C57BL/6 mice were exposed to 7.5 Gy of total body irradiation (TBI) or 13 Gy of total abdominal irradiation (TAI) in this study. After adjustment, D-gal was intraperitoneally injected into mice at a dose of 750 mg/kg/day. Survival rates, body weights, histological experiments and the level of the inflammatory factor IL-1β were observed after TBI to investigate radiation injury in mice. Feces were collected from mice for 16S high-throughput sequencing after TAI. Furthermore, fecal microorganism transplantation (FMT) was performed to confirm the effect of D-gal on radiation injury recovery. Intraperitoneally administered D-gal significantly increased the survival of irradiated mice by altering the gut microbiota structure. Furthermore, the fecal microbiota transplanted from D-gal-treated mice protected against radiation injury and improved the survival rate of recipient mice. Taken together, D-gal accelerates gut recovery following radiation injury by promoting the growth of specific microorganisms, especially those in the class Erysipelotrichia. The study discovered that D-gal-induced changes in the microbiota protect against radiation-induced intestinal injury. Erysipelotrichia and its metabolites are a promising therapeutic option for post-radiation intestinal regeneration.
Keywords: D-galactose (D-gal); Radiation injury; fecal microbiota transplantation; gut microbiota.
© The Author(s) 2022. Published by Oxford University Press on behalf of The Japanese Radiation Research Society and Japanese Society for Radiation Oncology.
Figures
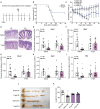
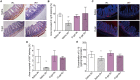
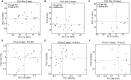

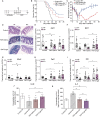

Comment in
-
D-galactose might protect against ionizing radiation by stimulating oxidative metabolism and modulating redox homeostasis.J Radiat Res. 2023 Jul 18;64(4):743-745. doi: 10.1093/jrr/rrad046. J Radiat Res. 2023. PMID: 37312584 Free PMC article. No abstract available.
Similar articles
-
Caloric restriction alleviates radiation injuries in a sex-dependent fashion.FASEB J. 2021 Aug;35(8):e21787. doi: 10.1096/fj.202100351RR. FASEB J. 2021. PMID: 34320242
-
Gut microbiota-derived indole 3-propionic acid protects against radiation toxicity via retaining acyl-CoA-binding protein.Microbiome. 2020 May 20;8(1):69. doi: 10.1186/s40168-020-00845-6. Microbiome. 2020. PMID: 32434586 Free PMC article.
-
Dysbiosis of Gut Microbiota Is Associated With the Progression of Radiation-Induced Intestinal Injury and Is Alleviated by Oral Compound Probiotics in Mouse Model.Front Cell Infect Microbiol. 2021 Oct 25;11:717636. doi: 10.3389/fcimb.2021.717636. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34760714 Free PMC article.
-
Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites.Biomed Pharmacother. 2021 Jul;139:111654. doi: 10.1016/j.biopha.2021.111654. Epub 2021 May 3. Biomed Pharmacother. 2021. PMID: 33957563
-
The Effects of Ionizing Radiation on Gut Microbiota, a Systematic Review.Nutrients. 2021 Aug 29;13(9):3025. doi: 10.3390/nu13093025. Nutrients. 2021. PMID: 34578902 Free PMC article.
Cited by
-
Efficacy and Mechanism of Quercetin in the Treatment of Experimental Colitis Using Network Pharmacology Analysis.Molecules. 2022 Dec 24;28(1):146. doi: 10.3390/molecules28010146. Molecules. 2022. PMID: 36615338 Free PMC article.
-
Insights into Gastrointestinal Redox Dysregulation in a Rat Model of Alzheimer's Disease and the Assessment of the Protective Potential of D-Galactose.ACS Omega. 2024 Feb 27;9(10):11288-11304. doi: 10.1021/acsomega.3c07152. eCollection 2024 Mar 12. ACS Omega. 2024. PMID: 38496956 Free PMC article.
-
Exploratory Study of Gastrointestinal Redox Biomarkers in the Presymptomatic and Symptomatic Tg2576 Mouse Model of Familial Alzheimer's Disease: Phenotypic Correlates and Effects of Chronic Oral d-Galactose.ACS Chem Neurosci. 2023 Nov 15;14(22):4013-4025. doi: 10.1021/acschemneuro.3c00495. Epub 2023 Nov 6. ACS Chem Neurosci. 2023. PMID: 37932005 Free PMC article.
-
D-galactose might protect against ionizing radiation by stimulating oxidative metabolism and modulating redox homeostasis.J Radiat Res. 2023 Jul 18;64(4):743-745. doi: 10.1093/jrr/rrad046. J Radiat Res. 2023. PMID: 37312584 Free PMC article. No abstract available.
-
Sweet regulation - The emerging immunoregulatory roles of hexoses.J Adv Res. 2025 Mar;69:361-379. doi: 10.1016/j.jare.2024.04.014. Epub 2024 Apr 15. J Adv Res. 2025. PMID: 38631430 Free PMC article. Review.
References
-
- Bentzen SM. Prevsenting or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer 2006;6:702–13. - PubMed
-
- Fliedner TM, Graessle D, Meineke V et al. Pathophysiological principles underlying the blood cell concentration responses used to assess the severity of effect after accidental whole-body radiation exposure: an essential basis for an evidence-based clinical triage. Exp Hematol 2007;35:8–16. - PubMed
-
- Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta oncologica (Stockholm, Sweden) 1996;35:659–63. - PubMed
-
- MacNaughton WK. Review article: new insights into the pathogenesis of radiation-induced intestinal dysfunction. Aliment Pharmacol Ther 2000;14:523–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

